Figure 8.1 Stage I: Intraretinal neovascularization.
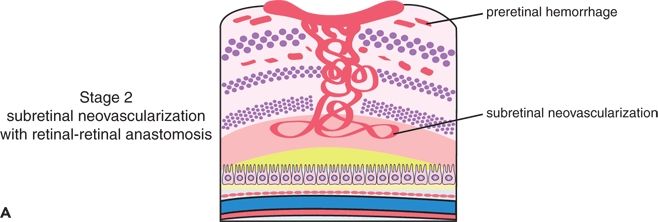

FIGURE 8.2 A. Stage II: subretinal neovascularization with a retinal–retinal anastomosis. B. Stage II: subretinal neovascularization with a serous PED.
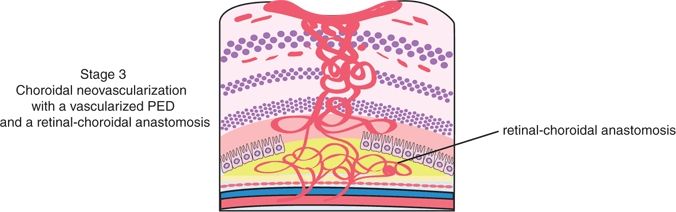
Figure 8.3 Stage III: choroidal neovascularization with a vascularized PED and a RCA.
Stage I: Intraretinal Neovascularization
Capillary proliferation within the retina, originating from the deep capillary plexus in the paramacular area, is the earliest manifestation detected in any patient with RAP. In this stage, there is usually a nodular mass of angiomatous tissue in the middle and inner retina. As this lesion progresses, there is a predominantly vertical extension of the IRN to the anterior and posterior boundaries of the retina. The neovascularization also may show a lateral extension of the vessels in an irregular stellate configuration. This is characterized by projections of capillaries tangentially and obliquely from the primary neovascularized area, resembling the appearance of a sea urchin. One or more prominently dilated perfusing arterioles or draining venules could be seen early in this stage, communicating with the core of the IRN. Evidence also demonstrates a retinal–retinal anastomosis (RRA). Some medium-sized capillaries projecting from the core of angiomatous proliferation have been noted to communicate with the larger, dilated inner retinal vessels. A few intraretinal hemorrhages and intraretinal edema could be seen clinically surrounding the IRN. The FA reveals a focal area of intraretinal staining with an indistinct border corresponding to the IRN and surrounding intraretinal edema. ICG reveals a focal area of intense hyperfluorescence within the retina or a so-called hot spot at the site of the IRN. Matsumoto et al. (20) showed with SD-OCT the origin of the IRN in nine eyes with untreated RAP. Retinal edema was always observed around the IRN in eyes with stage I. The frequency of retinal edema around the IRN and the low incidence of serous retinal detachment (SRD) suggest the intraretinal origin of neovascularization in RAP. The IRN was outside the avascular fovea in all eyes and appeared as a highly reflective mass that originated at the outer plexiform layer (OPL) and extended to the deeper retinal layers. In eyes at stage I, the highly reflective mass at the OPL corresponding to IRN disappeared, but drusenoid PED remained unchanged after repeated intravitreal bevacizumab (IVB) in SD-OCT, which lends further support to the postulation of intraretinal origin of RAP. The disruption of the RPE beneath the IRN is often seen in stage I. Although Freund et al. (11) interpreted the disrupted RPE as intraretinal invasion of CNV in early-stage RAP, it may reflect the drusenoid PED with attached IRN.
Stage II: Subretinal Neovascularization
Stage II is present when the IRN extends posteriorly beyond the photoreceptor layer of the retina into the subretinal space, forming SRN. At this point, there is a broader, tangential vascular proliferation under the retina. There is also a localized neurosensory retinal detachment, an increase in the intraretinal edema, and multiple small intraretinal hemorrhages that extend circumferentially to the limits of the macular detachment, but not beyond. Although there could be a slight thickening of the surface of the retina from the neovascularization, new vessels do not extend detectably into the preretinal space. As the RAP expands within the retina, and in particular beneath the retina, the dilated perfusing or draining retinal vessels could be seen clinically to decussate posteriorly toward the SRN. In stage II, a clear RRA could be seen, with a perfusing retina arteriole and draining venule communicating in some eyes with a “hairpin loop” within the core of the SRN. An associated serous PED also could be seen as the SRN reached or fused with the RPE. In FA, the SRN appears to have a small area of classic CNV predominantly within the occult lesion. The ICG angiogram reveals a hot spot at the site of the neovascularization within and beneath the retina. There is also some late extension of leakage in the retina from the IRN. Although this stage of classification assumes the presence of new subretinal vessels originating from the retinal vasculature, a choroidal vascular component could not be ruled out on the basis of the clinical and angiographic examinations. At this stage, SD-OCT shows major retinal edema around the IRN in comparison to stage I. SD-OCT can also show hemorrhagic or nonhemorrhagic PED, although Matsumoto et al. (20) demonstrated that no hemorrhagic PED was observed in the six eyes with stage II disease with PED. RRA and disrupted RPE beneath the IRN are also present. SRD is less frequent.
Stage III: Choroidal Neovascularization
A more common and definitive documentation of stage III is made when there is clinical and angiograph evidence of a vascularized PED or a predisciform scar. The ICG angiogram, however, is most helpful in determining the presence of CNV: Only the vascular components—IRN, SRN, and CNV—will be hyperfluorescent, and they can be distinguished as contrast is enhanced from the hypofluorescent exudative spaces. Identification of a clearly distinct and documentable RCA is rare. In a few cases, a connection between the two circulations could be identified, constituting an RCA. Otherwise, it can only be assumed that there is a communicating network of vessels between the two circulations.
A dilated retinal venule is particularly conspicuous in stage III, as perfusion of the SRN and CNV appears to be predominantly from the choroidal circulation with notable drainage into the retina. In these cases, the retinal and subretinal neovascularized components are noticeably smaller in lateral dimensions, compared to the more aggressive CNV beneath a distinctly larger vascularized PED. In fact, some patients have only a small area of IRN and SRN cascading above a large, placoid area of CNV. In a study by Krebs et al. (21) comparing the morphologic findings of the SD-OCT (Cirrus) to the time-domain OCT (Stratus), the authors reported the presence of RPE breaks only in eyes with RAP stage III. In OCT, the detection of a band of tissue protruding in the PED may be indicative of an accompanying CNV when FA or ICG fails to demonstrate it.
With the advent of new technology such as the SD-OCT, Freund et al. (22) do not see a value in the current staging system for RAP (or type 3 neovascularization), as it was based on observations of eyes with relatively long-standing lesions and does not accurately describe the evolution of these lesions. They argue that the advent of SD-OCT has helped explain one of the confusing aspects of this entity.
In the past, it was difficult to understand how a PED could occur in an eye with an RCA because, with the older time-domain OCT systems, one rarely saw evidence of vascular tissue bridging the space beneath the PED. These observations led some investigators to conclude that the neovascularization must have originated within the neurosensory retina. They believe that the RCA does persist after the elevation of the RPE monolayer in the form of type 1 vessels lining the undersurface of the RPE that maintain the connection to the choroidal circulation. These vessels are more readily detected with SD-OCT as hyperreflective material directly beneath the elevated hyperreflective RPE band.
TREATMENT
A range of alternative treatment options have been reported in case series with limited evidence of efficacy, from intravitreal treatments alone or, in combination with laser, to surgical excision of neovascularization in RAP, with variable success rates (16,23–38). Unfortunately, no randomized controlled data have been published to date. Treatment studies include thirty observational studies that had more than 3 months of follow-up and seven prospective studies. The main treatment options included inhibitors of vascular endothelial growth factor (anti-VEGF agents), photodynamic therapy (PDT) alone or in combination with intravitreal triamcinolone (IVTA) or anti-VEGF agents, focal laser, transpupillary thermotherapy (TTT), and surgical ablation alone or in combination with other agents. A few studies investigated the response of various stages of RAP, but the numbers are limited, and the prognosis (depending on the stage when treatment is started, the sooner the better) is currently considered to be poor.
Anti-VEGF Agents
These factors are thought to have a strong effect on RAP because an overexpression of VEGF is known to be the trigger of the deep retinal neovascularization in this condition (8). All three anti-VEGF agents available for treatment of neovascular AMD (pegaptanib sodium (39), ranibizumab, and bevacizumab) have demonstrated, in most of the studies, the capability to reduce vascular leakage and improve the visual outcome in patients with RAP. The protocols used for treatment in the available studies varied between them, and most investigators evaluated effect of as-needed dosing schedule based on visual acuity (VA) and retinal morphology. The VA recordings were converted to Early Treatment Diabetic Retinopathy Study (ETDRS) letters for analysis of mean change in visual acuity, resulting in +4 ETDRS letters at 3 months, +1.5 ETDRS at 6 months, and +3 ETDRS at 12 months. The change in VA was associated with a decrease in central retinal thickness. Nevertheless, persistence of activity of lesion was noted in nearly all patients at the end of follow-up (30,40–45). Only one eye experienced injection-related inflammation, which subsequently resolved with appropriate treatment (42). From the studies available, bevacizumab had the best response in all stages of RAP lesion with maximum efficacy at 3 months, sustained over 12 months. However, the results should be interpreted with caution as most of the studies used bevacizumab and did not include RAP stage, lesion size, and location. The sample sizes were also small.
Laser Photocoagulation
Direct laser photocoagulation of the neovascular process is a standardized treatment for extrafoveal CNV (46). The most appropriate modality of laser beam delivery for RAP lesions is unknown, although several investigators have used this treatment modality in extrafoveal RAPs. Focal laser ablation of RAP lesions was described previously by Hartnett et al. (6). Direct treatment with focal laser stabilizes most of the RAP lesion, although there is no statistical significant in visual outcome (28,29,47,48). In twelve eyes treated by Bottoni et al. (47) by retinal feeder vessel treatment, a closure rate of 25% was noted at the end of 23.4 months. As with other retinal vascular pathologies treated with feeder vessel treatment, reperfusion of the occluded vasculature is common. The same group reported 9/24 eyes achieving complete closure after a grid laser treatment. Using modified TTT and modified diode laser (two consecutive, superimposed, subthreshold spots of 3 and 1.2 mm), Bottoni et al. (47) achieved a closure rate of 40% with scarring previously reported following TTT, which has limited success. The use of ICG imaging allows differentiation of the intraretinal and subretinal components of neovascularization associated with stage II RAP lesions and selective ablation of only the intraretinal component of the neovascularization. FA is limited in its ability to detect RAP lesions for laser treatment.
Two studies provided the outcome of the combination of laser photocoagulation to the hot spot on ICG combined with IVTA (Krieglstein et al. (48) and Roth et al. (29)). Concomitant use of IVTA may also decompress the PED when present and suppress the neovascular drive, as observed by Krieglstein et al. (48) and Roth et al. (29). However, the effect of focal laser treatment is short lived. This effect could be due to either incomplete treatment with previous focal laser or early treatment for reperfusion leading to a prolonged effect. In addition, the use of micropulse laser may reduce associated collateral damage (29). In micropulse diode laser treatment, the laser energy is delivered with a series of repetitive short pulses (typically of 0.1–0.3 ms) so the duration of exposure is typically 0.1 to 0.5 second, and thus, there are minimum heat transfer and damage to overlying neural retina and limited collateral damage.
Photodynamic Therapy
It is difficult to predict stabilization of RAP neovascular lesion with PDT application because PDT targets CNV and not retinal vessels, the source of neovascularization in RAP. In addition, the risks of acute retinal tears are higher in RAP when associated with PED (24). Treatment with PDT was FA (and ICG) guided, and the standard protocol for PDT recommended by the verteporfin in photodynamic therapy (VIP) study group was used in all studies. Cumulative evidence from observational studies on PDT alone in RAP indicates a mean change of VA of −2 ETDRS letters at 3 months, −10 ETDRS letters at 6 months, and −1 ETDRS letters at 12 months.
Combination Treatment of PDT with Other Agents
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


