Anterior uveitis
Granulomatous
Non-granulomatous
Intermediate uveitis
Posterior and panuveitis
Retinal vasculitis
Multifocal serpiginoid choroiditis
Choroidal tubercles/tuberculoma
Subretinal abscess
Tubercular optic neuropathy
Papillitis
Neuroretinitis
Optic nerve granuloma
Endophthalmitis and panophthalmitis
Anterior Uveitis
This is typically granulomatous though the nongranulomatous form has also been described. Granulomatous uveitis is identified by presence of mutton-fat keratic precipitates and iris nodules (Koeppe and Busacca) and may be associated with broad posterior synechiae (Fig. 15.1).


Fig. 15.1
Tuberculous anterior uveitis showing ‘mutton-fat’ keratic precipitates and broad posterior synechiae
Intermediate Uveitis
TB can account for nearly half the cases of intermediate uveitis in high-endemic countries [10]. It presents as chronic, low-grade inflammation associated with vitritis, and ‘snowballs’ in vitreous cavity. ‘Snow-banking’ is less commonly seen. Long-standing disease may lead to cystoid macular edema, complicated cataract, elevated intraocular pressure, epiretinal membrane, and peripheral retinal neovascularization.
Posterior and Panuveitis
The most common manifestations of tubercular posterior uveitis in ophthalmic practice are retinal vasculitis and multifocal serpiginoid choroiditis. Different manifestations of posterior uveitis may co-exist in the same or opposite eye (Fig. 15.2). The posterior uveitis can be accompanied by generalized intraocular inflammation (panuveitis), anterior, and intermediate uveitis.
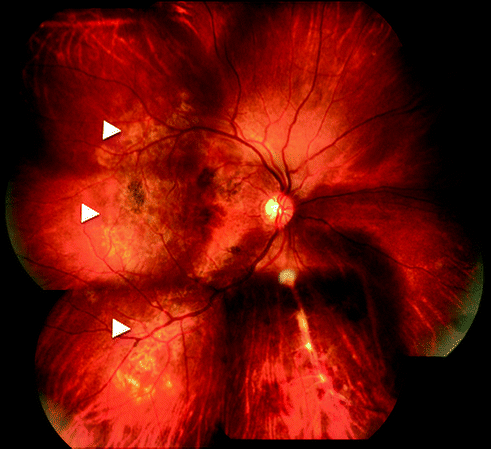

Fig. 15.2
Fundus photograph of right eye showing multifocal serpiginoid choroiditis (arrowheads) associated with retinal vasculitis and hemorrhages along inferonasal quadrant
Retinal Vasculitis
Tubercular retinal vasculitis typically involves the retinal veins, i.e., it causes retinal periphlebitis. It has two distinguishing features that can help in making a clinical diagnosis in high-endemic countries: presence of healed or active choroiditis patches usually overlying blood vessels and large areas of capillary non-perfusion (on fluorescein angiography) in segments drained by the involved veins (Figs. 15.3a, b and 15.4). Common complications include macular edema, retinal neovascularization, vitreous hemorrhage, and tractional retinal detachment.
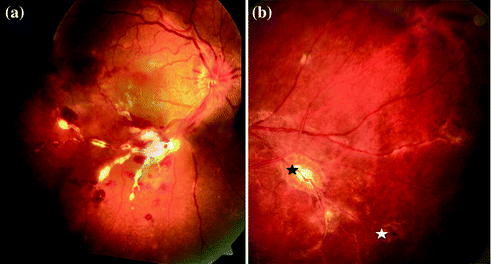
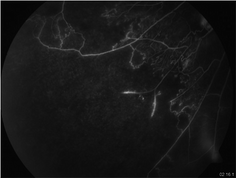

Fig. 15.3
a Fundus photograph of right eye showing retinal periphlebitis associated with massive perivascular exudation, in the inferotemporal quadrant. Retinal hemorrhages and macular edema are also seen. b Fundus photograph of inferotemporal quadrant of left eye showing active (black asterix) and healed (white asterix) choroiditis patches along blood vessels

Fig. 15.4
Fluorescein angiogram of inferotemporal quadrant of an eye with retinal vasculitis showing extensive areas of capillary non-perfusion and early retinal neovascularization
Eales’ disease is a related form of retinal periphlebitis, associated with recurrent vitreous hemorrhage. Polymerase chain reaction (PCR)-based analysis of ocular tissue samples from Eales’ disease patients has linked this condition to TB [11].
Infectious Multifocal Serpiginoid Choroiditis (MSC)
This is a chronic recurrent inflammation of the retinal pigment epithelium and inner choroid that shows central healing and active margins progressing in an ameboid fashion (Fig. 15.5a, b). Three patterns have been described: multifocal lesions that progress to confluent, diffuse choroiditis, lesions that are diffuse at presentation and a mixed variety (between opposite eyes) [12].
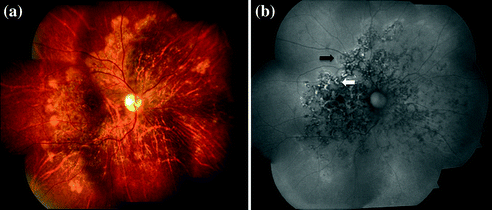

Fig. 15.5
a Fundus photograph of right eye showing large confluent area of serpiginoid choroiditis with central healing and active temporal margins b fundus autofluorescence of the same eye showing ill-defined halo of hyperfluorescence (black arrow) corresponding to the active lesions and dark ring of hypofluorescence (white arrow) appearing around early healing lesions. Multiple skip lesions along the nasal quadrants are also seen
The following characteristics distinguish MSC from classical serpiginous choroiditis: endemic population/travel to endemic area, multifocality, presence of vitritis, lesions originating at macular, dense pigmentation at center of lesions, and immunological/radiological evidence of systemic TB [13]. In TB non-endemic populations, other infective etiologies like herpes viruses and syphilis can present with features of MSC. However, in some the infectious agent cannot be detected even after extensive investigations including qPCR and retina-choroidal biopsy. Such cases remain as idiopathic.
Choroidal Tubercles/Tuberculoma
These may be solitary or multiple and are typically associated with active pulmonary/extrapulmonary TB. As such, they are more likely to be encountered in general hospitals than in ophthalmic practice. The lesions heal into atrophic patches with variable pigmentation. Occasionally, the tubercle may grow into a large solitary mass that is then called a tuberculoma, that need to be distinguished from neoplastic lesions; benign, or malignant (Fig. 15.6).
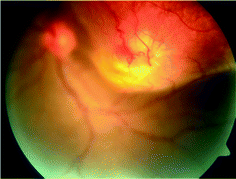

Fig. 15.6
Fundus photograph of left eye showing large mass lesion in superotemporal quadrant extending on to the macula. An area of retinal angiomatous proliferation is seen at the apex of the lesion
Subretinal Abscess
These are large yellowish subretinal lesions that occur due to liquefaction of caseous material in large tuberculous granulomas. They usually respond well to antituberculous therapy (ATT), healing with atrophy and variable pigmentation.
Tubercular Optic Neuritis
Theses may range from papillitis and neuroretinitis to optic nerve granuloma [14]. They are usually associated with some form of uveitis. Most cases recover well after appropriate therapy.
Endophthalmitis and Panophthalmitis
These present acutely with hypopyon and dense vitritis. Subretinal or choroidal abscess can be present that may burst into the vitreous cavity. Unlike the previously mentioned clinical manifestations, acid-fast bacilli may be demonstrated in aqueous or vitreous samples in such cases.
Pathogenesis
The pathogenesis of ocular TB in the form of intraocular inflammation/uveitis has long been debated. The identification of M. tuberculosis in enucleated eyes and amplification of mycobacterial DNA from ocular fluid samples suggest that this condition results from an inflammatory response to the bacteria in ocular tissues [15, 16]. Animal models have shown that mycobacteria are disseminated to the eye from pulmonary foci, as in other cases of extrapulmonary TB [17]. It has been suggested that mycobacteria remain sequestered in the RPE in a dormant form for long periods and on their activation they lead to recurrent inflammation [18]. Most recently, it has been shown that RPE cells are better able to control growth of M. tuberculosis than macrophages, which helps them survive longer in the presence of infection [19].
However, ocular TB is also paucibacillary, as noted in a large series of 42 patients with histopathologically proven ocular TB [20]. Thus the organism is rarely found in ocular tissues. This has led many authors to believe that ocular TB results from immune reaction to tubercular antigens released from extraocular foci. Such a phenomenon is not seen in any other form of TB and has not been validated for ocular TB.
Ocular Imaging
Although ocular TB is a clinical diagnosis in the majority of patients, imaging studies can be helpful in assessing disease activity, and evaluating associated complications of intraocular inflammation.
Fundus Photography
Serial fundus photography can be very useful in documenting disease progression as well as identifying small lesions that may be missed on routine examination.
Fluorescein Angiography
It is most useful in patients with retinal vasculitis in demonstrating areas of capillary non-perfusion and associated neovascularization (Fig. 15.4) [9]. In MSC, active margins show initial hypofluorescence and late hyperfluorescence while healed areas show transmission hyperfluorescence or blocked hypofluorescence depending on RPE atrophy or proliferation. It can also help in the diagnosis choroidal neovascular membranes that may complicate MSC.
Fundus Autofluorescence
It has emerged as a quick imaging tool for monitoring the course of MFC lesions [21]. Typically, acute lesions show an ill-defined halo of hyperautofluorescence. With healing, a dark outer rim of hypoautofluorescence appears, that progressively increases to occupy the entire lesion.
Optical Coherence Tomography (OCT)
It also shows characteristic changes in MFC lesions [22]. In spectral-domain optical coherence tomography (OCT), acute lesions show hyperreflective areas in outer retina and RPE with no back-scattering from inner choroid (Fig. 15.7). As the lesions heal, choroidal reflectance increases and the outer retina/RPE shows transition from hyperreflectivity to knob-like elevations and finally atrophy. OCT is also useful in documenting macular pathology like cystoid macular edema and epiretinal membranes.
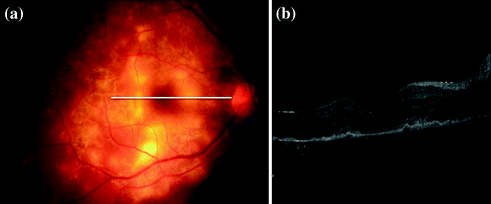

Fig. 15.7




a Fundus photograph of right eye showing multifocal serpiginoid choroiditis b spectral-domain optical coherence tomography of same eye showing hyperreflectivity of retinal pigment epithelium and outer retinal layers corresponding to active lesions, with minimum back scattering from underlying choroid
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


