Adapted from Finger PT, Berson A, Ng T, Szechter A. Ophthalmic plaque radiotherapy for age-related macular degeneration associated with subretinal neovascularization. Am J Ophthalmol. 1999;127(2):170–177.
Tear Film Disruption
Studies have described epiphora or transient conjunctival irritation and dry eye syndrome following radiotherapy (35,49). The symptoms may result from radiation damage to the lacrimal and meibomian glands, the conjunctiva itself, or its goblet cells (53,54). Deficiencies in any of the tear-film components (lipid layer, aqueous layer, mucinous layer) can result in unstable tear film, leading to dry eye symptoms, with secondary changes of the corneal and conjunctival surface. These effects are usually transient, typically lasting for a few months (55).
Cataract
The crystalline lens is the ocular structure with the highest susceptibility to radiation (48), with a reported threshold of 2 Gy (46). Lens opacities may develop within months or years, depending on the dose administered (56).
Radiation Optic Neuropathy
The threshold for clinically observable radiation damage to the optic nerve is reported to be greater than 55 Gy (19,57), with patients typically presenting after 8 to 16 months and very occasionally after 3 years (58). It is believed to be caused by damage to the vascular endothelial cells at the level of the choriocapillaris. This leads to vascular occlusions and vascular insufficiency of the optic nerve, which is then unable to meet the metabolic demands of the damaged tissue (59). Patients present with an anterior optic neuropathy that may manifest as papillitis and optic atrophy, characterized by initial disk swelling, hemorrhage, and cotton-wool spots (60). The prognosis is poor, often with a final visual acuity of less than 20/200, and many eyes become completely blind (61).
Radiation Retinopathy
Radiation retinopathy is an occlusive microangiopathy, secondary to endothelial cell loss and capillary closure. It leads to the formation of small, dilated collateral channels that bypass the area of ischemia and assume a telangiectatic-like form (62). It was first described in 1933 following the use of radon seeds to treat retinal tumors (63). Patients developed retinal inflammation and degeneration, with exudates, hemorrhages, retinal pigment epithelium changes, and optic disk edema. The retinopathy was slowly progressive with a delayed onset, typically 6 months to 3 years after radiation treatment, with the severity dependent on the total dose of radiation administered (64). It is believed that the retina can usually tolerate doses up to 35 to 50 Gy, beyond which radiation retinopathy may occur (57,65,66).
Clinically, radiation retinopathy has an appearance similar to that of diabetic retinopathy, with the development of microaneurysms, followed by retinal hemorrhages, telangiectatic vessels, capillary nonperfusion, infarcts of the nerve fiber layer, and vascular obliterations at the level of the choriocapillaris (67–69). The earliest clinical features are discrete foci of occluded capillaries and irregular dilatation of the neighboring microvasculature. Fundus fluorescein angiography can confirm the extent of capillary dropout and the general capillary competence at this early stage (70). Indocyanine green angiography at an early stage shows atrophy of the retinal pigment epithelium and choriocapillaris, and eventually, the larger choroidal vessels became nonperfused (71). Vision loss is caused by macular edema, exudation, and retinal ischemia. In the late stages, secondary ischemic complications may lead to proliferative retinopathy, neovascularization, and subsequent vitreous hemorrhages, rubeosis iridis, and tractional retinal detachments (72–74). Patients who develop proliferative radiation retinopathy have a poor prognosis, with most achieving only 20/200 vision or worse after 6 years (75).
Studies using newer treatment modalities have described a non–vision-threatening form of radiation retinopathy (76–78). This is characterized by a nonprogressive, nonproliferative retinopathy and often favorable visual outcomes; indeed, it has been reported that the visual outcomes were better than those in participants who did not develop radiation retinopathy, at least over the 2- to 3-year time frames of the studies that have reported to date (77,78). This suggests that some patients may be more sensitive to the effects or radiation—both its beneficial and deleterious effects (77). Key clinical findings were macular intraretinal hemorrhages and telangiectatic dilatations of the perifoveolar capillaries and small areas of capillary nonperfusion that are best detected using fluorescein angiography (Fig. 20.1) (78).



FIGURE 20.1 A. Color fundus photograph before treatment with epimacular brachytherapy, showing subfoveal CNV associated with subretinal hemorrhage. B. Color fundus photograph 19 months after the initial treatment. The image shows fibrotic CNV surrounded by small superficial hemorrhages. C. Fluorescein angiography showing telangiectatic dilatation of the perifoveal capillaries, consistent with mild, nonproliferative radiation retinopathy. The CNV appears inactive, with no signs of leakage. (Reprinted with permission from Navarro R, Bures-Jelstrup A; Vasquez LM, et al. Radiation maculopathy after epimacular brachytherapy for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Retin Cases Brief Rep. 2011;5(4):352–354.)
CURRENT RADIOTHERAPY TREATMENTS
There has been renewed interest in the use of radiation to treat wet AMD, with three methods of delivery undergoing clinical trials: epimacular brachytherapy, stereotactic radiotherapy, and proton beam irradiation (Table 20.2).
Table 20.2 SUMMARY OF THE EPIMACULAR BRACHYTHERAPY AND STEREOTACTIC RADIOTHERAPY CLINICAL TRIALS

Epimacular Brachytherapy
Unlike previous attempts using external beam radiation, epimacular brachytherapy was specifically developed to deliver intraocular radiation. The device (VIDION; NeoVista Inc., Fremont, CA) uses beta radiation from a strontium-90/yttrium-90 source. Beta radiation has a rapid decline in dose with increasing distance from the source (Fig. 20.2). This limits radiation exposure to a defined region, minimizing the risk of damage to adjacent normal tissue. The central macular lesion receives 24 Gy, the optic nerve receives 2.4 Gy, and the lens receives 0.00056 Gy (Fig. 20.3) (Table 20.2) (48,57,65,66,79–81).

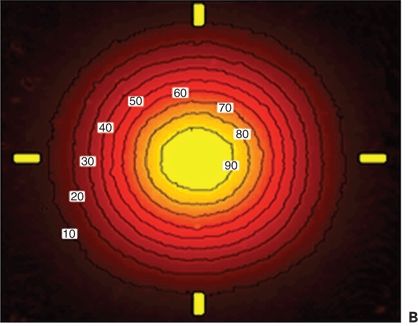
Figure 20.2 Dose attenuation with distance. A. Graph showing the attenuation in the dose of radiation delivered, in relation to distance from the source. In general, it can be seen that the dose is inversely related to the square of the distance from the probe. B. —Radiochromic Film Contour Plot for the strontium-90 radiation source. White boxes represent the percent of maximum dose. The yellow hash marks on the image correspond to the horizontal and vertical (top-down) dose rate profiles as displayed in the left image. The inside edge of each hash mark is equidistant to the radiation center of the source. (Courtesy of NeoVista.)


FIGURE 20.3 A,B. Computer-generated images showing the dose attenuation with distance from the probe to the retinal surface (red = 24 Gy) and in 0.1-mm increments (orange = 20 Gy; yellow = 17 Gy; light green = 14 Gy; dark green = 11 Gy; light blue = 10 Gy; and dark blue = 9 Gy). (Courtesy of NeoVista.)
The beta radiation used in epimacular brachytherapy is delivered via a pars plana vitrectomy (Fig. 20.4). Once the vitreous has been removed, the surgeon positions the probe over the CNV lesion (Fig. 20.5). A preoperative fundus fluorescein angiogram is used to ensure that the area of maximum dosage is directed over the area of greatest disease activity. The probe is held in position for approximately 4 minutes and then removed. Surgery is usually undertaken using local anesthetic in a day case setting.

Figure 20.4 Intraocular probe in the midvitreous cavity, prior to placement on the retinal surface. (Courtesy of NeoVista.)

Figure 20.5 Epimacular brachytherapy probe positioned on the retinal surface, during surgery.
In the initial published trials of epimacular brachytherapy, patients received an anti-VEGF injection at or near to the time of surgery and again 1 month later to treat any preexisting disease activity at the time of surgery. Thereafter, they had anti-VEGF therapy as needed, based on disease activity (79,82).
The combination of vitrectomy and radiation may be uniquely suited to the treatment of AMD. It has been proposed that removal of the vitreous increases the level of oxygen available to the inner layers of the retina via diffusion from the aqueous humor (83–85). A reduced oxygen tension may play a role in the initial CNV formation, and elevated oxygenation is likely to reduce VEGF levels (86). Increasing oxygenation in the treatment area at the time of brachytherapy may increase the formation of free radicals and therefore the double-stranded DNA breaks required to treat CNV lesions (87,88). The disadvantage of vitrectomy is that it may reduce the half-life of ranibizumab, as animal studies using other drugs indicate that vitrectomy may increase the rate of drug elimination from the vitreous (89–92).
CLINICAL TRIALS OF EPIMACULAR BRACHYTHERAPY
Two key studies provided the early data on the safety and efficacy of epimacular brachytherapy.
NVI-068
The first, NVI-068, was a nonrandomized multicentre feasibility study that recruited 34 treatment-naive participants in Mexico and Brazil. Subjects received either 15 Gy (eight participants) or 24 Gy (26 participants) of beta radiation. Twelve months after treatment, there were no radiation-related adverse events recorded. There was however a significant difference in ETDRS visual acuity between the two groups. In the 24-Gy group, the mean visual acuity improved by 10.3 letters (approximately two Snellen lines), while the 15-Gy group lost 1.0 letters (82).
NVI-111
The second trial, NVI-111, was a prospective, nonrandomized, multicentre study that enrolled 34 treatment-naive participants in Mexico and Brazil. Participants were treated with a single dose of 24 Gy epimacular brachytherapy and two injections of anti-VEGF therapy. The first injection was given either at approximately 10 days before surgery or at the conclusion of epimacular brachytherapy. The second injection was given 1 month later. Thereafter, anti-VEGF therapy was administered monthly PRN. Twelve months after treatment, there were no reported cases of radiation retinopathy. ETDRS visual acuity showed a mean improvement of 8.9 letters (approximately two Snellen lines), with 91% maintaining vision (defined as a loss of fewer than 15 ETDRS letters) and 68% having stable or improved visual acuity (improved by ≥0 letters) (79). Approximately three-quarters of participants required no further anti-VEGF therapy over the first year. By comparison, the Comparison of AMD Treatment Trials (CATT) study found that participants required an average of 7.7 anti-VEGF injections over this interval (4).
Subsequent review of the NVI-111 participants at 24 months revealed a mean visual acuity of −4.9 ETDRS letters (approximately one Snellen line), but the authors attributed this loss of vision to the development of cataract in the phakic participants. This appeared to be supported by the 36-month data that revealed a final mean visual acuity of +3.9 ETDRS letters, and a mean of only three retreatment injections during this time (76). There was one case of nonproliferative retinopathy identified in the 36-month report, with relatively subtle microvascular changes (telangiectatic vessels, vessel beading, and microaneurysms). The changes were detected in the parafoveal region of a patient who had no adverse effect on visual acuity (improved one line from baseline) and who remained stable at 43 months of follow-up with only one additional anti-VEGF injection administered at the first month after brachytherapy (76).
The promising results of these pilot studies led to the larger studies of epimacular brachytherapy.
MERITAGE
The Macular EpiRetinal brachytherapy in Treated AGE-related macular degeneration (MERITAGE) study (ClinicalTrials.gov Identifier: NCT00809419) was a multicentre, noncontrolled Phase II study evaluating the safety and efficacy of epimacular brachytherapy in participants who required persistent anti-VEGF injections. MERITAGE enrolled 53 participants from five sites in the United States, Israel, and the United Kingdom. The objective was to investigate if visual acuity could be maintained while reducing the demand of retreatment with anti-VEGF therapy. Unlike the preceding studies, MERITAGE enrolled participants who were already receiving treatment with ranibizumab or bevacizumab. In addition, participants were required to meet minimum prior injection criteria to be eligible, namely three rescue injections in the 6 months preceding enrollment or five rescue injections in the 12 months preceding enrollment.
Prior to enrollment, the participants had a mean duration of disease of 28 months and had received an average of 12.5 anti-VEGF treatments, with one participant receiving a maximum of 38 prior injections. As a result, this study selectively recruited participants with the most active disease, who might otherwise be predicted to have a poor outcome. This differs from previous studies on radiotherapy that recruited treatment-naive disease, where the case mix may have been more typical. Because participants have already commenced treatment, the MERITAGE study did not expect to show visual gains, unlike studies that recruit untreated disease.
The MERITAGE study reported that after 12 months of follow-up, the mean change in visual acuity was −4.0 ETDRS letters (approximately one Snellen line) with a mean of 3.2 anti-VEGF injections. The proportion of participants maintaining vision (losing fewer than 15 ETDRS letters) was 81%, and the rate of administration of anti-VEGF injections had reduced from 0.45 injections per participant per month prior to enrollment to 0.27 injections per participant per month over 12 months (47).
Microperimetry and indocyanine green angiography were performed on all participants at one of the MERITAGE recruiting sites, to determine if the radiotherapy causes reduced retinal sensitivity or choroidal ischemia. The site reported similar results to previous investigations using strontium-90 (93), with no loss of retinal sensitivity or evidence of choroidal damage after 12 months. There appeared to be a positive dose response, with a statistically significant correlation between proximity to the radioactive source and improvement in retinal sensitivity (Fig. 20.6). This response was most significant within the neovascular lesion area. This suggests that the benefit of epimacular brachytherapy is due, at least in part, to the effect of radiation, and not some other confounding variable, such as the vitrectomy procedure, as the areas that received the higher doses demonstrated the greatest functional improvement. The authors speculated that it might be possible to offer a more customized dose delivery, rather than offering a standard dose of 24 Gy, regardless of the lesion type, size, or topography (94).



Figure 20.6 Determining the relationship between radiation dose and retinal sensitivity. A. The change in sensitivity was calculated for areas within and outside the AMD lesion area. This boundary was defined using the fundus fluorescein angiogram images and included all components of the AMD lesion, including any hemorrhage, fibrosis, or leakage. This was then superimposed onto the microperimetry fundus photograph so that data points could be mapped. B. Positioning of the probe was planned prior to surgery, to ensure delivery of the maximum dose to the most active part of the AMD lesion. Probe position was recorded at the end of the operation by marking the FFA. Once the FFA was overlaid on the microperimetry field, it was possible to calculate the distance from the probe for each sensitivity data point. It was thus possible to correlate the change in retinal sensitivity to the dose of radiation received. C. The epimacular brachytherapy probe is etched with a cross that corresponds to the position of the radioactive pellet within the probe. The retina beneath this cross receives the maximum 24-Gray dose.
A subsequent report analyzed the optical coherence tomography (OCT) and fundus fluorescein angiograms of the MERITAGE participants at 12 months. OCT center thickness increased by 50 μm over this time frame, but the median increase was only 1.8 μm, reflecting the fact that the mean increase was driven by a minority of eyes with relatively large increases (95). The angiographic lesion size was substantially larger than most other trials, and this would be expected to result in worse visual outcome (96).
Both the OCT and visual outcomes suggested that classic lesions responded better than occult lesions. Over half of the participants had occult lesions (with no classic) with a mean center thickness increase of 62 μm and loss of 5 ETDRS letters (one Snellen line). Minimally classic lesions had an increase of 51 μm and lost 6 ETDRS letters. Predominately classic lesions decreased by 43 μm and gained 2 ETDRS letters (95).
The adverse events reported in the first 12 months of the MERITAGE study were mainly related to the development of postvitrectomy cataract, with no radiation retinopathy identified. The study will however monitor participants for 3 years, as radiation retinopathy may occur at a later stage (47).
In summary, the MERITAGE study recruited patients with active disease, who were losing vision despite frequent anti-VEGF therapy. It demonstrated stabilization of vision in a majority of participants and a reduced frequency of reinjection. Subgroup analysis suggested classic lesions were the most responsive.
CABERNET
The CNV Secondary to AMD Treated with BEta RadiatioN Epiretinal Therapy (CABERNET) study (ClinicalTrials.gov Identifier: NCT00454389) was the first multinational, pivotal, randomized controlled trial evaluating the safety and efficacy of epimacular brachytherapy in treatment-naive participants. CABERNET enrolled 457 participants across 45 sites in the United States, Europe, Israel, and South America. The 302 participants in the epimacular brachytherapy (24 Gy) treatment arm had two mandatory ranibizumab injections. The 155 participants in the control arm received ranibizumab injections following a modified PIER protocol, which included three initial monthly injections followed by injections every 3 months. Participants in both arms were seen on a monthly basis, and rescue therapy was permitted according to protocol-specific retreatment criteria.
At the 24-month end point, the CABERNET study failed to meet its primary outcomes using a predefined 10% noninferiority margin and 97% confidence interval, indicating that in treatment-naive participants, the use of epimacular brachytherapy was not shown to be noninferior to anti-VEGF monotherapy, in terms of vision maintenance (77). The epimacular brachytherapy group received a mean of 6.2 injections of ranibizumab treatments and had a mean change in visual acuity of −2.5 ETDRS letters, while the control group received a mean of 10.4 injections and had a mean change in visual acuity of +4.4 ETDRS letters (approximately one Snellen line) (77).
In the epimacular brachytherapy arm, 42% of participants received no injections after 12 months and only one injection up to 24 months, with a mean gain of 3.3 ETDRS letters; 27% of participants received no rescue injections throughout the 24-month course of the study and achieved a gain of 5.7 ETDRS letters. These results suggested there were some lesions that were particularly sensitive to the treatment (77).
Post hoc analysis, at a 20% noninferiority margin and a 95% confidence interval, found that epimacular brachytherapy was noninferior for lesions smaller than 3.5 disk areas and for classic or minimally classic lesions, as judged by the investigator. It was observed that the large occult lesions drove the mean visual acuity results down. The authors speculated that the edge of these larger lesions may have received a subtherapeutic dose due to the rapid tapering of the treatment dose from the center, which, although advantageous in terms of safety, may lead to parts of larger lesions being undertreated. Furthermore, positioning of the probe over the area of greatest disease activity may have been difficult with occult lesions, due to their indistinct boundaries and their variable shape, which makes it difficult to define a geometric centre (77).
CABERNET demonstrated that epimacular brachytherapy had an acceptable safety profile over a 2-year interval. The most frequent adverse event reported was cataract, occurring in 40% of the participants who were phakic at enrollment, and which was most likely related to the vitrectomy rather than the radiotherapy. There were ten cases (3%) of suspected nonprogressive, nonproliferative, radiation retinopathy, but interestingly, these participants tended to have a positive clinical outcome, with a mean gain in vision of 4.4 ETDRS letters (approximately one Snellen line) and a mean of only two rescue injections over 2 years. Further follow-up is planned, as radiation retinopathy can occur beyond a 24-month window.
RETINAL ANGIOMATOUS PROLIFERATION
Although there is little clinical evidence to date, theoretically, epimacular brachytherapy may be well suited to the treatment of retinal angiomatous proliferation (RAP). These lesions often have rapid and aggressive growth (97,98), and assuming then that they are highly mitotic, they should be more susceptible to the effects of the ionizing radiation. Further, their small size and easily defined centre means it is possible to accurately administer the maximum dose (24 Gy) to almost the entire lesion.
A single case of RAP treated with epimacular brachytherapy has been reported, with a positive clinical outcome. Preoperatively, the patient had received eight ranibizumab injections over a 10-month period and had active leakage during angiography. At 12 months after epimacular brachytherapy, she had gained 16 ETDRS letters (approximately three Snellen lines), with resolution angiographic leakage (Fig. 20.7) with only one further anti-VEGF injection over 21 months (99).

Figure 20.7 Treatment of a RAP lesion: The top row shows fundus photographs, early fundus fluorescein angiography (FFA), late fundus fluorescein angiography, indocyanine green angiography (ICG), and optical coherence tomography (OCT) results at baseline (preoperative). The second row shows results at 6 months after epimacular brachytherapy. The third row shows results at 12 months. The bottom row shows the results at further follow-up of 21 months. The FFAs show persisting late staining of fibrous tissue but a loss of active leakage seen at baseline, following treatment with epimacular brachytherapy. ICG fails to show any deleterious effects of radiation on the choroidal circulation. OCT shows a marked reduction in intraretinal fluid that persists over 21 months. (Reprinted with permission from Petrarca R, Nau J, Dugel P, et al. Epimacular brachytherapy for the treatment of retinal angiomatous proliferation. Retin Cases Brief Rep. 2012;6(4):353–357.)
IDIOPATHIC POLYPOIDAL CHOROIDAL VASCULOPATHY
The efficacy of epimacular brachytherapy in cases of idiopathic polypoidal choroidal vasculopathy (IPCV) (100,101) is unknown, but it is possible that IPCV may be amenable to radiation, and furthermore, these lesions often show a suboptimal response to anti-VEGF injections. Conversely, radiation has been associated with the development of IPCV (102).
There have been two identified cases of IPCV treated by epimacular brachytherapy, both within the MERITAGE study. In both cases, the lesions were seen using indocyanine green angiography, with one lesion located at the macular and the other in a peripapillary location (94). Prior to enrollment in the study, the participants had received a mean of 21 injections over a mean of 29 months. At 12 months following treatment with epimacular brachytherapy, the mean change in vision was +3.0 ETDRS letters, with a mean of only three anti-VEGF treatments.
MERLOT
The Macular EpiRetinal Brachytherapy versus Lucentis Only Treatment (MERLOT) study (ClinicalTrials.gov Identifier: NCT01006538) is a pivotal, multicenter, randomized controlled trial in the United Kingdom, recruiting participants who have already commenced anti-VEGF therapy. It has completed recruitment with a total of 363 participants who were randomized in a 2:1 ratio, to either epimacular brachytherapy (24 Gy) with as-required ranibizumab or as-required ranibizumab monotherapy. The MERLOT study, like MERITAGE, targets participants requiring regular anti-VEGF injections. The rationale is that there are limited surgical resources, and these resources are best directed to those who have not fully responded to ranibizumab therapy or whose response is short-lived. These participants have the most to gain from a therapy that may reduce their frequency of anti-VEGF retreatment (103). The 12-month results are expected in early 2013.
Stereotactic Radiotherapy
More recently, investigators have revisited external beam therapy, using a technique called Stereotactic Radiosurgery or Stereotactic Radiotherapy. The IRay system (Oraya Therapeutics, Newark, CA, USA) uses a low-voltage x-ray source that does not require the same degree of radiation shielding as the early linear accelerators. The system is designed to fit in a standard clinical environment and runs off a 220 to 240 V wall socket, without the need for room shielding or expensive safety precautions (Fig. 20.8) (80).

Figure 20.8 The photograph shows the IRay system used to deliver stereotactic radiotherapy. The patient and operator site on opposite sides of a lead-lined glass screen. (Courtesy of Oraya.)
The system is designed to overcome the traditional disadvantages of external beam therapy by dividing the dose into separate beams that pass into the eye via different locations and overlap on the CNV lesion (104). The beams pass through the inferior pars plana region of the sclera, at the 5-, 6-, and 7-o’clock positions (Fig. 20.9). These overlap on the predicted foveal center, dispersing the scleral entry dose and minimizing exposure of the lens and optic nerve (80,104,105). The patient is secured in position with a head restraint that also contains a lead backing to prevent radiation traveling beyond the patient. Exposure to the lower lid is limited by a lid retractor. The operator is separated from the patient during treatment via a lead-lined glass shield, which allows the operator to monitor the patient (Fig 20.5) (80,106). The patient’s eye is then secured in position with a vacuum-coupled contact lens interface with suction; the system can detect any eye motion and stabilizes the eye during treatment (Fig. 20.10). The eye is then continually tracked during treatment, and an inbuilt safety feature interrupts the radiation treatment if the eye moves out of position. The system can treat most AMD lesions centered on the fovea, with an average accuracy of 0.6 mm and a precision of 0.4 mm (107,108).

Figure 20.9 Illustration of the trajectory of the stereotactic radiotherapy beams that pass through the pars plana, overlapping on the macula, and avoiding the lens and optic nerve. (Courtesy of Oraya.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


