Uveitis is an umbrella term for a variety of inflammatory eye conditions. It is a major cause of severe visual impairment worldwide and affects not only the uvea but also the retina, optic nerve, and the vitreous.1 The etiology may be idiopathic or secondary to various infectious, neoplastic, or autoimmune diseases. Currently, many imaging modalities, such as color fundus photography, fluorescein angiography (FA), indocyanine green angiography (ICGA), optical coherence tomography (OCT), and fundus autofluorescence (FAF) have been used for the diagnosis and follow-up of patients with uveitis.2,3,4,5,6,7,8 These imaging modalities can be used to identify and monitor the development of visually debilitating sequelae, such as vasculitis, retinal and choroidal neovascularization, and cystoid macular edema (CME).3,4,5,9 The gold standard techniques, FA and ICGA, are not perfect given they are invasive, involving the use of intravenous dye that can result in systemic side effects and rarely anaphylaxis.10,11,12
Unlike FA and ICGA, OCT angiography (OCTA) is a noninvasive, depth-resolved imaging modality that allows for the appreciation of spatial relationships of fundus vessels and enables detailed en face visualization of the retinal and choroidal vasculature separately without the risk of adverse effects associated with the administration of intravenous dye.13,14 OCTA uses signal decorrelation between consecutive transverse cross-sectional OCT scans.15 An OCTA image is computed by comparing, on a pixel-by-pixel basis, repeated B-scans acquired at the same retinal location in rapid succession. The rationale behind OCTA imaging is that in static nonmobile tissue, the reflected signal will be stationary, thus the repeated B-scans will be identical. Inside vasculature, moving erythrocytes cause a time-dependent backscattering of the OCT signal, so that the repeated B-scans are not identical.13,14,16 This change between the sequential B-scans can be processed to provide an OCTA scan. Areas of flow are noted as white and areas of no flow are seen as black. Previous studies have shown that the 3 × 3 mm OCT angiograms can visualize greater vascular detail compared to FA or ICGA.17,18 Importantly, OCT angiograms can also be viewed alongside corresponding structural en face and OCT B-scans, which can be used to visualize increased central retinal thickness and intraretinal cysts and correlate these structural findings to microvascular details.
14.2 Optical Coherence Tomography Angiography in Retinal Vasculitis
Retinal vasculitis is a sight-threatening complication that can be present in many systemic autoimmune, inflammatory, and infectious diseases such as sarcoidosis, Behçet’s disease, lupus, multiple sclerosis, birdshot chorioretinopathy, cytomegalovirus, and many others.19,20,21,22,23 On clinical examination, retinal vasculitis presents as vascular sheathing, cotton wool spots, retinal hemorrhages, and vein occlusions that can lead to secondary retinal ischemia, neovascularization of the retina and optic disc, and CME.24 These features are often well demonstrated by FA, which has been the gold standard to evaluate disease activity and subsequent adverse events. This imaging modality can easily identify leakage from the vasculature wall secondary to a breakdown of the blood–retinal barrier, areas of ischemia, and retinal and optic disc neovascularization.25
The retinal capillary network is arranged in distinct layers and are connected by perpendicularly positioned vessels.26 The capillaries from the superficial retinal plexus are located predominantly within the ganglion cell layer, whereas the capillaries from the deep retinal plexus are located at the outer boundary of the inner nuclear layer, with a smaller intermediate retinal capillary plexus at the inner margin of the inner nuclear layer. Previous studies showed that these two retinal capillary plexuses could be disproportionately affected by a retinal vascular disease, and the deep vascular plexus often presents a more prominent decreased flow.18,27 However, since dye-based angiography is only able to visualize the retinal vasculature using two-dimensional imaging, independent evaluation of those plexuses was only possible with the advent of the depth-resolved capability of OCTA. Patients with retinal occlusion who are often noted to have areas of ischemic capillary nonperfusion on FA have recently been correlated with areas of retinal nonperfusion on OCT angiograms.28 In occlusive vasculitis, OCTA imaging highlights that the vessels can become tortuous, narrowed, and focally dilated. Truncated vessels can also be present on OCT angiograms with abrupt interruptions and focal terminal dilations at the site of the occlusion. Vascular looping and telangiectatic vessels that are often obscured by dye leakage on FA can also be easily visualized with OCTA.29
Another important feature of OCTA is the ability to automatically evaluate some quantitative vascular features such as vessel density and flow indices ( ▶ Fig. 14.1).30,31 Additionally, the combination of blood flow analysis obtained by OCT angiograms to the structural en face and OCT B-scans makes this imaging modality a useful tool for evaluating progression of disease and response to treatment in retinal vasculitis.

Fig. 14.1 Quantitative analysis of the vascular density using optical coherence tomography angiography (OCTA) of the left eye of a 46-year-old female patient with retinal vasculitis secondary to Behçet’s disease. (a) Color fundus photography shows diffuse retinal atrophy associated with vascular sheathing and tortuosity. (b) Yellow dashed frame identifies the corresponding 3 × 3 mm area imaged on OCTA. (c) En face OCTA segmented at the level of the superficial retinal plexus. (d) Corresponding OCT B-scan segmentation of the superficial plexus with decorrelation signal overlay. (e) Quantitative analysis of the retinal thickness (µm) and vascular density (%). (f) Superficial capillary plexus map shows a mild decrease of the vascular density (39.49%).
The disadvantage of OCTA in retinal vasculitis is the inability to detect vascular sheathing, an important sign that can be easily seen on dye-based angiography. This vascular leakage is not visualized by OCTA for two reasons. First, because retinal vasculitis is associated with leukocytes extravasation into the extravascular space,32 not erythrocytes, and this leaking fluid does not strongly backscatter the incident OCT beam. Second, the relatively low leakage flux in retinal vasculitis means that even if the fluid did appreciably backscatter the OCT beam, the flux would not be detectable with OCTA, which is typically sensitive to backscatters with speeds in the millimeter per second range ( ▶ Fig. 14.2).

Fig. 14.2 Multimodal imaging of the left eye of a 46-year-old female patient with retinal vasculitis secondary to Behçet’s disease. (a) Fluorescein angiography (FA) shows a diffuse background hyperfluorescence associated with vascular sheathing. (b) Yellow dashed frame identifies the corresponding 3 × 3 mm area imaged on optical coherence tomography angiography (OCTA). The yellow arrow points the area of vascular sheathing. (c,d) En face OCTA segmented at the level of the superficial and deep retinal plexuses, respectively. OCTA did not reproduce the same FA finding. (e,f) represents corresponding OCT B-scans segmentation of the superficial and deep plexuses, respectively, with decorrelation signal overlay.
14.3 Optical Coherence Tomography Angiography in Chorioretinal Inflammations
The greatest advantage of OCTA compared to previous imaging modalities in posterior uveitis is the ability to noninvasively monitor these patients for potential complications such as choroidal neovascularization (CNV), which can be associated with severe visual loss.
14.3.1 White Dot Syndromes
White dot syndromes (WDS) are a group of inflammatory chorioretinal diseases characterized by the presence of yellowish-white lesions. Those lesions can affect the choroid, retinal pigment epithelium (RPE), and retina.33 The WDS spectrum includes birdshot chorioretinopathy, serpiginous choroiditis, multifocal choroiditis (MFC), punctate inner choroidopathy (PIC), and acute zonal occult outer retinopathy (AZOOR). Symptoms may include mild to severe decrease in visual acuity that can often be associated with visual field defects and photopsia. The use of multimodal imaging has guided the diagnosis and treatment through the use of FAF, FA, ICGA, and structural OCT B-scans.5,6,34
Previous reports have shown that OCTA could detect choriocapillaris flow impairment in birdshot chorioretinopathy besides the aforementioned retinal vascular disorders.35 In acute serpiginous choroiditis, markedly increased thickness and decreased circulation in the choroid has been described.36 OCTA can detect reduced choriocapillaris flow in these patients. However, this finding needs to be cautiously evaluated since the sub-RPE plaques present in this phase can block the OCT signal, leading to a misdiagnosis of flow impairment. In patients with Zone 3 (choroidal atrophy) AZOOR, OCTA depicts a choriocapillaris impairment. In MFC and PIC, OCTA becomes crucial as it allows for the early detection of secondary CNV.
14.3.2 OCT Angiography for Secondary Choroidal Neovascularization
Perhaps one of the most important applications of this imaging modality is the possibility to early detect CNV as an adverse event of posterior uveitis (toxoplasmosis, serpiginous choroiditis, MFC, PIC, etc.; ▶ Fig. 14.3 and ▶ Fig. 14.4).37 This sight-threating complication demands a correct and early diagnosis for prompt intraocular anti–vascular endothelial growth factor treatment.38 Evaluation of CNV using OCTA has been extensively described and is one of the most important applications of this modality.16,39,40 Depending on the clinical presentation, OCTA sensitivity to visualize abnormal vascular network may vary. The CNV complex can often be identified as abnormal choroidal vessels that correspond to a distinct, high-flow, tangled irregular filamentous vascular network in the outer retinal, choriocapillaris, or in both layers.41 When a massive hemorrhage, exudate, or fibrotic tissue is present, OCTA signals can be blocked, decreasing visualization. Because CNV secondary to posterior uveitis is rarely associated with massive subretinal hemorrhages that limit penetration of OCT signal, the abnormal vascular network may be identified with a screening OCTA in such cases.
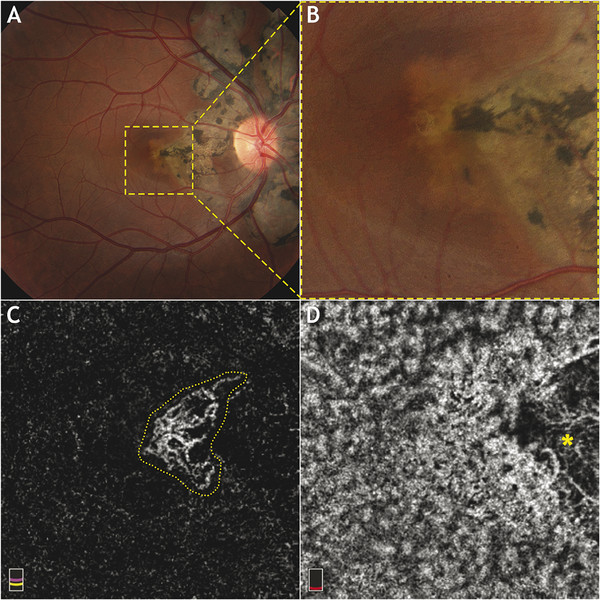
Fig. 14.3 Multimodal imaging of the right eye of a 30-year-old female patient with choroidal neovascularization (CNV) secondary to serpiginous choroiditis. (a) Color fundus photography shows a peripapillary chorioretinal atrophy. (b) Yellow dashed frame identifies the corresponding 3 × 3 mm area imaged on optical coherence tomography angiography (OCTA). (c) En face OCTA segmented at the level of the outer retina shows a distinct and filamentous CNV (yellow dotted line). (d) En face OCTA segmented at the level of the choriocapillaris shows an area of absence of flow that corresponds to the chorioretinal atrophy area (yellow asterisk).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


