




FIGURE 11.1 Type 1 CNV in a 72-year-old patient with AMD (A). OCT imaging sub-RPE neovascularization with subretinal fluid (B). ICG angiography shows a hyperfluorescent “plaque” of neovascularization (black arrow) (C–F).
The third group combined both hot spots and plaques in one lesion.
ICG angiography has proven to be particularly useful in detecting neovascular components in PEDs. Unlike on FA, serous PEDs appear comparatively hypofluorescent on ICG angiography because only minimal ICG leakage occurs beneath the serous detachment (27). Vascularized components of a PED, on the other hand, are hyperfluorescent. Patients with vascularized PEDs are further classified by the location of the CNV as seen on ICG. Small areas of hyperfluorescent CNV at the margin or outside and indenting the PED are termed notch (Fig. 11.2) (40). Intraretinal proliferations (INRs) associated with PEDs are suggestive of RAP lesions or type 3 lesions, which may advance to chorioretinal anastomosis (41).





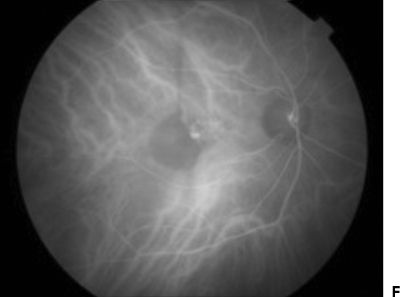


Figure 11.2 A 79-year-old patient with type 1 neovascular AMD. FA shows a vascularized pigment epithelial detachment (white arrowhead) with a neighboring serous retinal PED (white arrow) (A–D). ICG angiography shows hypofluorescence of the serous PED (black arrowhead) with a small area of hyperfluorescent CNV (“notch,” black arrow) (E–H).
Using the above classification system, Guyer et al. (42) found that out of 680 patients with occult CNV, 22% had localized lesions, previously undetected by FA and potentially amenable to laser therapy, the treatment of choice at that time. Still, about 1% of patients with occult CNV on FA did not show any changes on ICG angiography (39).
Polypoidal Choroidal Vasculopathy
Currently, the most common indication for the use of ICG angiography in AMD is the detection of PCV. Although PCV is most common in Asian populations where it has been described in as many as 50% of AMD patients (43–45), it can be found in all ethnic groups (43,46,47). Missing the diagnosis potentially leads to treatment failures given the less robust response of these lesions to anti-VEGF therapy (48). Active PCV presents as an inner choroidal vascular network ending in aneurysmal bulges. At times, these bulges can be seen as red-orange, spheroid-like structures with indirect ophthalmoscopy. More frequently, they are obscured by hemorrhages and/or the overlying retina and RPE. ICG can be used to identify and characterize the vascular abnormality with high sensitivity and specificity (1,46,49–57). In the early phase of the ICG angiogram, PCV appears as a distinct, branching vascular network. Soon after the lesion is first visible on ICG angiography, small hyperfluorescent “polyps” emerge within the lesion actively leaking into the surrounding hypofluorescent area and creating increasing hyperfluorescence. In the late phases of the ICG angiogram, the dye disappears from the polypoidal vascular structure (“washout”) (Fig. 11.3).



FIGURE 11.3 Color photography of a patient with AMD: The typical orange polypoidal lesions are almost undetectable (arrow) (A). Midphase ICG angiography reveals hyperfluorescent “hot spots” representing individual polyps, the hallmark of PCV (arrow) (B). The polyps leak into the surrounding hypofluorescent area, creating patterns of increasing hyperfluorescence. A “washout” effect is seen as the dye disappears from the polypoidal vascular structure in the late phases of the ICG angiogram (arrow) (C).
Wide-field angiography, which currently combines SLO-based imaging devices with a handheld wide-angle viewing lens system (e.g., Staurenghi 230 SLO retina lens), allows detection of choroidal abnormalities in the outer periphery. Using this new technique, Mantel and coworkers found that two-thirds of patients with peripheral exudative hemorrhagic chorioretinopathy have polyp-like structures and share many features with PCV.
Classic or Type 2 Choroidal Neovascularization
Classic (type 2) CNV has variable appearance on ICG imaging, with most lesions showing at both the early and late phases or, less commonly, only at the late phase as well-defined, hyperfluorescent structures. However, there is a subset of patients that present with ill-defined or no detectable hyperfluorescence, confirming FA as the method of choice for imaging this type of lesion (58–60). Most authors have found that ICG angiography is the most valuable form of imaging in studying details of occult (type 1) (17,39,58,61,62) and RAP (type 3) lesions (1,41,63–66).
Retinal Angiomatous Proliferation
RAP, also known as type 3 neovascularization, is a distinct subgroup of neovascular AMD lesions in which intraretinal vascular proliferations are the characteristic manifestation. The neovascularization, which extends into the outer retina and subretinal space, is typically accompanied by dilated retinal vessels, hemorrhages (preretinal, intraretinal, and subretinal), and exudates. One or more of the related compensatory retinal vessels may perfuse and drain the neovascularization, forming a retinal–retinal anastomosis (RRA) in more advanced stages. On FA, RAP lesions show the same indistinct staining as seen in occult CNV; therefore, most cases require the use of ICG angiography to make the diagnosis (41). RAP lesions reveal a focal area of early and intense hyperfluorescence (“hot spot”) on ICG.
In the later phases of the ICG study, the leakage originating from the intraretinal neovascularization extends within the deep layers of the retina. Koizumi et al. (67) were able to show that the majority of patients with early-stage RAP also present with abnormal choroidal filling. In later stages of RAP, ICG angiography allows differentiation between the serous PED and the neovascular complex connecting the INR with the choroidal vasculature (Fig. 11.4) (68,69).






FIGURE 11.4 Color photograph (A) and red-free photograph (B) of a patient with RAP: Note the small hemorrhage indicating the area of INR (arrow). FA shows a small area of hyperfluorescent proliferation (C) with late leakage (D). ICG angiography shows a focal INR (E) with increasing hyperfluorescence in the late phase (F).
Although standard ICG angiography is excellent in detecting RAP lesions, detection rates may be even higher when using high-speed or dynamic ICG videoangiography provided by SLO-based technology imaging systems (66). This technique allows for visualization of the blood flow from the retinal artery to the intraretinal complex to the retinal venule in real time.
ICG: THERAPEUTIC APPLICATIONS
When ICG angiography was first adapted for diagnosing retinal and choroidal pathologies, clinicians and scientists focused on expanding the spectrum of neovascular lesions amendable to thermal laser photocoagulation. Over time, diagnostic and therapeutic indications evolved, using ICG-guided and ICG-enhanced feeder-vessel photocoagulation as well as ICG-mediated photodynamic therapy (PDT). Most recently, Flower et al. (70) pioneered a technique injecting ICG-loaded erythrocyte ghost cells to visualize retinal capillary and choriocapillaris hemodynamics.
ICG-Guided and ICG-Enhanced Feeder-Vessel Photocoagulation
In some cases of CNV, distinct vessels within the choroid that seem to form the basic source of blood flow to the neovascular lesion, the so-called feeder vessels (FVs), can be identified in the early phase of the angiographic study, especially using video sequences with an SLO-based imaging system. Analysis of the images permits identification of the FVs, based on the dye-filling pattern of the choroidal blood vessels in the vicinity of the CNV (71,72). Selective treatment of the FVs is applied by either using an argon laser (72) or using an ICG bolus injection with a diode laser at 810 nm (“ICG enhanced”) (73). The underlying principle is that successful closure of the FV(s) can shut down the perfusion of the entire neovascular network and thereby indirectly obliterate the CNV. While initially success rates of 40% to 70% were reported (72,74,75), most clinicians have stopped investigating “feeder-vessel” treatment. Today, thermal laser photocoagulation is largely reserved to treat singular eccentric polypoidal CNV lesions that are not responsive to conventional medical therapy (1).
ICG-Mediated Photodynamic Therapy
ICG has photochemical properties, which allow for it to be used as a photosensitizer. Activated in the near-infrared spectrum, it was thought to be of special use for choroidal and sub-RPE lesions, which can be imaged with the same dye used for treatment. First described by Reichel, “indocyanine green dye-enhanced diode laser photocoagulation” showed variable results, from successful obliteration of CNV to significant vision loss post treatment (76). Due to increasing interest in PDT for AMD in the late 1990s and early 2000s, clinicians explored again the use of ICG as a more affordable substitute for the photosensitizer verteporfin (Visudyne™, QLT Inc., Vancouver, Canada) (77–83). Although some of the reported results seemed promising, the treatment never gained wider popularity due to lack of sufficient dosimetry data, the concerns of additional thermal damage, and the introduction of anti-VEGF therapy.
ICG Angiography and Anti-VEGF Therapy
Since the advent of anti-VEGF therapy for neovascular AMD, the use of ICG angiography has shifted, focusing on identifying lesion subtypes, which may show a less favorable or no response to anti-VEGF therapy. This group is mostly comprised of PCV lesions either mimicking other AMD subtypes or as a sign of vascular maturation seen mainly in ill-defined CNV lesions (48).
Chronic central serous chorioretinopathy is another important differential diagnosis to make with the help ICG angiography (1). Especially with more advanced age, the typical funduscopic changes of CSC appear more similar to AMD. ICG angiography permits visualization of the characteristic choroidal hyperpermeability presenting as multifocal areas of patchy hyperfluorescence (84) (Fig. 11.5). When approaching a treatment strategy beyond anti-VEGF drugs, these areas can be targeted with standard or reduced fluence PDT. ICG angiography–guided PDT has been shown to be very successful and frequently yields better results than standard anti-VEGF therapy in this subset of patients (85). It is also important to note that the success of PDT correlates directly with the degree of vascular hyperpermeability/hyperfluorescence seen on ICG (86).





FIGURE 11.5 FA of a patient with chronic central serous chorioretinopathy: Note the pooling in the small PED (arrow) as well as the multiple window defects due to RPE atrophy (A,B). ICG angiography shows areas of increased fluorescence delineating choroidal hyperpermeability. The PED presents as early hypo- and later hyperfluorescence (arrow) (C,D). OCT imaging shows a PED with subretinal fluid (E).
Summary
Although less utilized than before the advent of anti-VEGF therapy, ICG angiography continues to play a crucial role in diagnosing specific subtypes of age-related macular degeneration such as RAP and PCV lesions as well as identifying unsuspected underlying conditions such as CSC. In these forms of AMD, the combined use of ICG angiography in conjunction with SD-OCT is the best way to understand the exact composition of the neovascular lesion and to guide appropriate treatment.
REFERENCES
1.Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011;151(5):745–751, e741.
2.Fox IJ, Brooker LG, Heseltine DW, et al. A tricarbocyanine dye for continuous recording of dilution curves in whole blood independent of variations in blood oxygen saturation. Proc Staff Meet Mayo Clin. 1957;32(18):478–484.
3.Fox IJ, Wood EH. Applications of dilution curves recorded from the right side of the heart or venous circulation with the aid of a new indicator dye. Proc Staff Meet Mayo Clin. 1957;32(19):541–550.
4.Cherrick GR, Pothier L, Dufour JJ, et al. Immunologic response to tetanus toxoid inoculation in patients with hepatic cirrhosis. N Engl J Med. 1959; 261(7):340–342.
5.Cherrick GR, Stein SW, Leevy CM, et al. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600.
6.Geeraets WJ, Berry ER. Ocular spectral characteristics as related to hazards from lasers and other light sources. Am J Ophthalmol. 1968;66(1):15–20.
7.Kogure K, David NJ, Yamanouchi U, et al. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol. 1970;83(2):209–214.
8.Flower RW, Hochheimer BF. Clinical infrared absorption angiography of the choroid. Am J Ophthalmol. 1972;73(3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


