Cuticular Drusen
Cuticular drusen or basal laminar drusen (BLD) were described by Gass in 1977 (5). Patients with BLD have numerous, small, uniformly sized, round, slightly raised, yellow, subretinal lesions on ocular fundus examination. These lesions are grouped into small clusters and usually span the macula between the arcades (Fig. 9.1A). The drusen are hyperfluorescent during fluorescein angiography and fade in later-phase frames (Fig. 9.1B). The lesions may develop a yellowish vitelliform material anterior to RPE. As it progresses, it clears and develops a marked or minimal geographic atrophy. The vitelliform material can block choroidal fluorescence in early stages of fluorescein angiography and stain in the later phase. The staining in later frames may mimic the appearance of type 1 occult CNV.


FIGURE 9.1 A. Basal laminar drusen. B. Fluorescein angiography showing starry-night pattern.
Initially, the term BLD was used to distinguish these drusen from drusen associated with AMD (6). Subsequent histologic analysis demonstrated that both types of drusen were indistinguishable. Both the BLD and the drusen associated with AMD are located between the basement membrane of the RPE and the inner collagenous layer of the Bruch’s membrane (7). Furthermore, both types of drusen were shown to be histologically indistinct using light and electron microscopy. This entity should not be confused with the histopathologic terms “basal lamellar deposits” or “basal linear deposits.” Although the visual prognosis for cuticular drusen is better than the visual prognosis for patients with AMD, the possibility of CNV exists in both diseases and is associated with worse visual acuity outcomes (8).
Central Serous Chorioretinopathy
This idiopathic disease is most common in Caucasian, Hispanic, and Asian patients between the second and fifth decade of their lives. More often, it is diagnosed in patients with type A personality, high stress levels, or use of corticosteroids (inhaled, topical, or systemic). CSC clinically presents as a sensory retinal detachment that may also be associated with RPE serous retinal detachment. The diagnostic challenge is significant for older age groups with changes in the RPE due to multifocal serous detachments (9,10). In some cases, the formation of “gutter–like” distribution of RPE atrophy in the absence of drusen may help make the diagnosis (Fig. 9.2).


Figure 9.2 Central serous chorioretinopathy. A. Fundus photograph, right eye, showing RPE changes from CSC. B. Fundus photograph, left eye, showing RPE changes from CSC.
Patients with chronic or recurrent CSC may have decreased visual acuity as a result of RPE dysfunction, subretinal precipitates, and subretinal CNV. The presence of CNV and subretinal fluid in a patient with CSC may be difficult to distinguish from neovascular AMD. Diagnostic clues include absence of drusen, absence of hemorrhage, patches of RPE atrophy in a gutter–like distribution, and the presence of multiple pigmented epithelium detachments (Fig. 9.3).



Figure 9.3 Central serous chorioretinopathy. A. Fundus photograph, right eye, showing RPE changes from multifocal serous detachments. B. Fluorescein angiography showing leakage. C. OCT showing multifocal serous pigmented epithelium detachments and subretinal fluid.
Pattern Dystrophy
The term “pattern dystrophy” represents a group of related conditions inherited in an autosomal dominant fashion. These macular dystrophies are relatively benign disorders. They are characterized by an abnormality at the level of RPE with hyperpigmentation and variably shaped yellow or gray deposits (Fig. 9.4A and B). The patients are asymptomatic and usually have normal vision. The classic appearance of pattern dystrophy demonstrates the pigmented lesions in the shape of a butterfly. The lesions are clearly seen in the fluorescein angiography. Typically, the lesions are hypofluorescent with a surrounding hyperfluorescent rim (Fig. 9.4C and D). This configuration helps to differentiate them from soft drusen of AMD (11–13). Other pattern dystrophies that we need to have in mind are reticular dystrophy of Sjögren and macroreticular dystrophy.




FIGURE 9.4 A,B. Pattern dystrophy of the RPE, right and left eye. C. Fluorescein angiogram, right eye, reveals central hypofluorescence surrounded by hyperfluorescence. D. Fluorescein angiogram, left eye, showing central staining.
Chloroquine Toxicity
Chloroquine has high affinity with melanin and is deposited in the RPE. The toxic damage is initiated by the degeneration of the photoreceptors, with subsequent degeneration of other retinal cells and the RPE. RPE mottling and nongeographic atrophy may be seen in the macular area in patients with toxic changes. The absence of drusen and a positive history of drug ingestion are essential to make the diagnosis (14) (Fig. 9.5A and B).


FIGURE 9.5 A. Chloroquine toxicity. Note the lack of drusen and presence of retinal pigment epithelial changes similar to nonneovascular age-related macular degeneration. B. Fluorescein angiography of the same fundus.
Dominant Drusen
This entity is inherited in an autosomal dominant fashion, affecting individuals between the second and third decade of their lives, and it is best observed on fluorescein angiography. Drusen may become confluent after the fourth decade. Initially, patients are asymptomatic. Eventually, central vision loss correlates with macular involvement, and it can be distinguished from AMD only on the basis of family history (15) (Fig. 9.6).



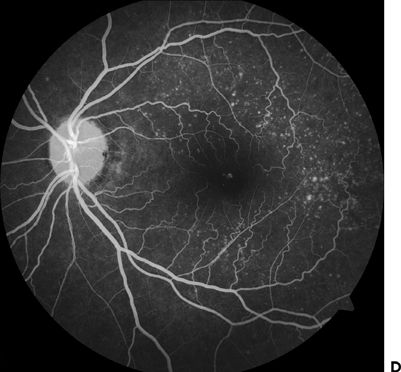
FIGURE 9.6 A,B. Fundus photo showing dominant drusen syndrome. C,D. Fluorescein angiography of the same eyes.
Central Areolar Choroidal Atrophy
The mean age at onset of visual loss is the fourth decade of life, with subsequent gradual deterioration in visual acuity. At early stages, the disease presents no symptoms associated with symmetric fine mottled areolar depigmentation of the fovea. In later stages, the depigmented area develops a sharply demarcated geographic atrophy that involves photoreceptors, RPE, and choriocapillaris (Fig. 9.7). In the elderly patient, central areolar choroidal atrophy may be confused with AMD (16).


FIGURE 9.7 A. Central areolar RPE atrophy. B. Fluorescein angiography of the same fundus.
NEOVASCULAR AGE-RELATED MACULAR DEGENERATION
The term CNV refers to ingrown fibrovascular tissue from the choroidal circulation into or underneath the retina. This fibrovascular tissue can progress to a disciform scar and cause irreversible vision loss. Any pathologic process that disturbs the RPE and choroid, leading to the disruption of the Bruch’s membrane, can be associated with CNV (17). There are different causes of CNV; some of those more frequently found are age-related macular degeneration, ocular histoplasmosis, multifocal choroiditis, myopia, and trauma.
CNV has been divided in two types: type I and type II. Type I CNV is characterized by a neovascular growth between the inner and outer aspects of the Bruch’s membrane, and it is more frequent in AMD. In type II CNV, neovascular growth occurs within the subsensory retinal space. Type II CNV is more frequent in young patients (18–20). Clinically, we suspect the presence of age-related CNV, typically type I, when a patient older than 60 years complains of metamorphopsia or scotoma and the ocular fundus reveals fluid or blood in the subretinal space. An RPE detachment and intraretinal blood are also possible indicators of this condition. Intravenous fluorescein angiography and optical coherence tomography (OCT) are needed to evaluate the presence of CNV and are helpful in assessing the differential diagnosis of the causes of the CNV.
DIFFERENTIAL DIAGNOSIS OF NEOVASCULAR AGE-RELATED MACULAR DEGENERATION
There are multiple clinical entities that can mimic neovascular AMD (Table 9.2). We discuss in this section some of the most important entities.
Table 9.2 DIFFERENTIAL DIAGNOSIS OF NEOVASCULAR AMD

Retinal Arteriolar Macroaneurysms
Retinal arteriolar macroaneurysms are present in more than 75% of patients with arterial hypertension over 60 years old. Significantly fewer are associated with retinal vein occlusions. Clinical features of retinal arteriolar macroaneurysm include focal dilation of a retinal artery with hemorrhages in all anatomic compartments (subretinal, intraretinal, or vitreous), retinal edema, and circinate lipid exudates. When the hemorrhage or associated lipid extends into the macula, it may be confused with CNV. Fluorescein angiography findings will differentiate the two entities. Macroaneurysms will demonstrate focal hyperfluorescence in the early frames, with late staining of the damaged vascular wall. Often, an area of capillary nonperfusion surrounds the lesion. The neighboring vasculature may exhibit microaneurysms and intra-arterial collateral formation (21–23) (Fig. 9.8).


FIGURE 9.8 A. Retinal macroaneurysm. The presence of hemorrhage and exudates extending into the macula may lead to a diagnosis of CNV. B. Fluorescein angiography demonstrates the early filling of the macroaneurysm.
Vitelliform Dystrophy
The presence of a vitelliform material, as in adult-onset foveomacular vitelliform dystrophy, can mimic an occult CNV in the late phase of a fluorescein angiography. Also, vitelliform lesions in adults can have fundus features that can be mistaken for a pigmented epithelium detachment (24) (Fig. 9.9).


FIGURE 9.9 A. Vitelliform dystrophy. B. Fluorescein angiography of the same fundus. Unlike CNV, this material shows early hypofluorescence and a ring of irregular hyperfluorescence surrounding the hypo- or nonfluorescent area.
Acquired Juxtafoveal Telangiectasia
Juxtafoveal telangiectasia is a term that describes entities presenting with incompetence, ectasia, and/or irregular dilations of the capillary network affecting only the parafoveal region of one or both eyes. The term idiopathic juxtafoveolar retinal telangiectasias (IJFT) was coined by Gass and Oyakawa in 1982 (25). They proposed the first classification of these entities into four groups based largely on their clinical and fluorescein angiographic features. In 1993, Gass and Blodi (26) further updated this classification by subdividing IJFT into three distinct groups, 1, 2, and 3, based on demographic difference or clinical severity. In this section, we discuss two subgroups of IJFT that may resemble neovascular AMD, especially when pigmentary changes and fibrovascular scarring are present.
Group1B (unilateral idiopathic juxtafoveal telangiectasias) is congenital and found in males in the fourth decade of life. The fovea avascular zone may be significant smaller and almost completely vascularized (Fig. 9.10).



Figure 9.10 Idiopathic juxtafoveal telangiectasias (IJFT). A. Fundus photo showing IJFT. B,C. Fluorescein angiography demonstrating early and late frames of the same eye.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


