Fig. 16.1
Slit lamp images on presentation of right eye (a) and left eye (b) and on follow up of right eye (c) and left eye (d), demonstrating significant reduction in corneal neovascularization and lipid deposition primarily in the left eye over the course of 2 months
What Differential Diagnoses Do These Findings Suggest? What Additional History Would This Exam Prompt You to Obtain?
The differential diagnoses would include inflammatory and infectious causes of pediatric ocular surface disease. Infectious etiologies could include herpes simplex, although it most commonly presents unilaterally there are more severe cases with bilateral disease [1]. Allergic or immune causes include vernal keratoconjunctivitis, although vernal is more common in boys and is typically associated with giant papillae and shield ulcers [2]. From the description we know that the process is bilateral with involvement of the eyelids, conjunctivae, and corneas. The most likely diagnosis is phlyctenular keratoconjunctivitis in the setting of chronic blepharitis, which is very prevalent in children [3, 4].
There are a few more relevant historical questions to ask. You would want to know if this child has a history of oral cold sores and whether she has a history of rosacea. You would also ask if any history of contact lens wear. A history of recurrent chalazia is also consistent with the diagnosis of phlyctenulosis and frequently present.
Upon questioning her family reports that she has been diagnosed with rosacea but has no history of prior cold sores. She has never worn contact lenses. She does have a history of multiple styes in the past. Prior to our examination, she had been initiated on oral erythromycin 400 mg by mouth twice a day and ciprofloxacin one drop every 4 h in the left eye.
What Therapeutic Plan Would You Initiate for a Child of This Age Group?
The most important element to recognize is the significant inflammation. The severe vision impairment in the left eye along with the dramatic corneal appearance of stromal haze, lipid deposition, and angry neovascularization does make one concerned for possible destructive infectious processes, but antibiotics or antivirals alone will not treat this vision-threatening condition. Anti-inflammatory activity is necessary to dampen the immune reaction that is causing lipid deposition, loss of corneal clarity, and neovascularization. The best initial approach includes topical steroids and oral anti-inflammatory and antibiotic therapy appropriate for this age group in addition to eyelid hygiene (preferably with eyelid cleansers) with warm compresses.
Due to the severe ocular inflammation, bilateral nature, and vision loss, an aggressive therapeutic approach was taken with prednisolone acetate 1% one drop every 4 h in the left eye, one drop twice a day in the right eye, and continuation of the oral erythromycin. Cyclosporine 0.05% one drop twice a day to both eyes was prescribed. The ciprofloxacin was discontinued. She was asked to return in 3 weeks, and documentation with slit lamp photography was performed.
Why Continue with Oral Erythromycin But Not Topical Ciprofloxacin?
Oral erythromycin can be an effective systemic agent for ocular surface inflammation in the pediatric age group. The macrolides’ bacteriostatic properties have been found to inhibit production of pro-inflammatory cytokines and thus have been useful as an anti-inflammatory agent for the eye [5]. The challenges in compliance are tied to its requirement of multiple daily dosing.
Doxycycline , which is an inexpensive, effective option in adults and older children for ocular inflammation, has been generally contraindicated in children less than 9 years of age due to concerns of deposition of the drug in bone and teeth. However, it has been used safely in many children with manifestations of ocular rosacea with good control of their disease [6]. Due to the petite frame of this child and the other alternatives, we decided to defer the option of doxycycline for management of her disease until she is older.
Topical antibiotics are not necessary in this child because clinically her exam is consistent with inflammatory processes and there is no ulceration or epithelial breakdown to suggest ocular surface compromise with microbes. As discussed below, there may be a role for topical antibiotics to the lid (to reduce the microbial flora); however we prefer to start with systemic medications.
Initial response to the therapy was positive with significant reduction in overall ocular surface inflammation. She was unable to get the cyclosporine due to insurance coverage.
What Concerns Would You Have About Long-Term Therapy?
The goals of therapy include suppression of the ocular surface inflammation and resultant vision-threatening scars. However, one must recognize the significant side effects of the medications involved and carefully titrate to effect while also minimizing potential adverse effects. Topical steroid therapy is well known to elevate intraocular pressures, and children are more vulnerable to this problem than adults [7]. In addition, lenticular changes that can cause vision loss are also a potential concern. However, one must realize that when balancing the competing factors involved in visual preservation, corneal destruction and scarring can often be much more difficult to ultimately treat compared with the resources we have to mitigate intraocular pressure rises and cataracts. Corneal transplantation in children is fraught with challenges [8], especially in the postoperative period, and in an eye prone to inflammation the risk of graft rejection is increased.
Why Cyclosporine? What Is the Pediatric Experience?
Cyclosporine has become widely used to mediate the inflammatory component of dry eye and has been shown to increase tear production [9]. It is commercially available in a 0.05% formulation but can be compounded in a 2% formulation for more severe ocular surface disease. In a series of children with a mean age of 9 years old, the cyclosporine 2% was given four times a day for an average of 1 year with significant resolution of ocular surface inflammation. These children were selected due to poor response to topical steroids and oral antibiotics, and the topical cyclosporine was found to be well tolerated in this age group [10]. The steroid-sparing effect is clearly advantageous. Although it is not FDA approved for the treatment of phlyctenular disease in children, the literature confirms its utility for this indication as an off-label ophthalmic use.
What Oral Antibiotics Are Safe and Effective for Children with Ocular Surface Disease and Ocular Rosacea?
As discussed earlier, doxycycline and erythromycin have been used in children when age and dose appropriate for the control of ocular surface inflammation. If these agents are poorly tolerated or contraindicated, another option that has recently been highlighted for the pediatric population is azithromycin. Oral azithromycin, dosed 5 mg/kg/day with a single daily dose, has been shown to have effective anti-inflammatory effect on the ocular surface in children with phlyctenular disease [11]. It is commonly given at that dose for approximately 1 month and then given at half dose for another month. It is well tolerated, and the once a day dosing in our experience has been great for compliance in the pediatric population.
Azithromycin has also been utilized in its topical form to treat children with this disease. One formulation studied was azithromycin topical ophthalmic solution 1.5%, and in a series of 16 children ranging in age from 4 to 16 years old who had disease poorly responsive to other typical therapies, the majority of children were found to have tolerated the eye drops (two stopped therapy due to ocular irritation), and only one child required additional therapy for disease control [12].
What Other Considerations/Treatment Approaches Would You Have if This Child Was Within the Amblyopic Age Group?
Any ocular surface disease has the potential to cause scarring or vision-threatening opacities. In the amblyopic age group, it is critical to remember that early intervention is the key to preservation of vision. Therefore, when a child presents with corneal scarring or severe keratitis that is primarily unilateral or asymmetric, they should be assessed for the risk of development or presence of amblyopia. Sometimes this requires early patching or more aggressive therapy than one might institute in an adult with the same findings, so as to prevent deprivational amblyopia.
She continues to have recurrent episodes of ocular inflammation ( Fig. 16.2 ) in the left eye with deeper corneal neovascularization, despite topical and oral steroids, topical cyclosporine, and oral azithromycin .


Fig. 16.2
June 2014 Slit lamp images demonstrating recurrent robust keratoconjunctivitis causing aggressive deeper stromal corneal neovascularization in the nasal region of the left eye
What Would Be Your Next Therapeutic Approach to This Challenge?
Recalcitrant phlyctenular disease can be approached using localized therapy to the eyelids, which are often the source of the inflammation. Topical tacrolimus applied to the eyelids has been proven efficacious in reduction of ocular inflammation in these more challenging cases. Tacrolimus has been shown to have a potency 30 times more than cyclosporine. The formulation evaluated in the pediatric age group for this indication is 0.03% tacrolimus ointment to the lower fornices twice daily for a few weeks with good results and was well tolerated [13].
Our patient did not initially tolerate the tacrolimus preparation available, but we recommended dilution of the ointment with artificial tear lubricating gel, and she was able to tolerate it and achieve good effect. With institution of the above alternative therapy, the robust inflammation in her left eye became quiescent, and her vision measured 20/30 ( Fig. 16.3 ).
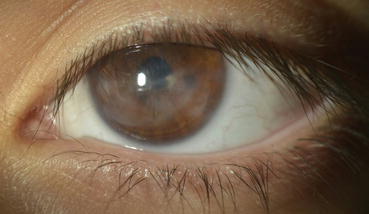

Fig. 16.3
Jan 2015: 15 months after treatment there is significant reduction of corneal haze and lipid deposition with evident control of ocular surface inflammation. Vision is 20/30 uncorrected
What Is the Underlying Cause of This Type of Ocular Surface Inflammation?
Phlyctenular keratoconjunctivitis is a common inflammatory ocular surface condition in children with many available therapies. In many literature series, the larger spectrum of blepharokeratoconjunctivitis, of which phlyctenular disease is a subset, represents the most common referral diagnosis for pediatric patients to ophthalmic referral centers. Phlyctenular disease has traditionally been associated with a hypersensitivity reaction of the ocular surface, namely, the cornea and the conjunctiva, to bacterial antigens. The most common agents implicated are tuberculosis, Staphylococcus, and chlamydial antigens, although other less common antigens can also trigger this disease process [14]. In patients with recalcitrant disease, it may be reasonable to check for tuberculosis (Quantiferon Gold). Phlyctenular disease can also occur in the setting of chronic eyelid inflammation, and therefore a multipronged approach to the ocular surface destruction, eyelid inflammation, and bacterial hypersensitivity is critical. Another condition that must be considered in recalcitrant disease is demodex. Diagnosis requires examining lashes under light microscope. Current treatments for demodex include eyelid scrubs with 50% tea tree oil or oral or topical ivermectin [15].
Vision-threatening and amblyogenic scarring can occur, especially since the average age of presentation of these children is reported to be around 6 years old [14–17]. Therefore, we must consider visual development in the context of therapeutic decisions and potentially involve pediatric ophthalmologists in the disease management process if the child is perhaps being managed by an internist or ophthalmic subspecialist who may not treat amblyopia as part of their daily practice.
Judy Chen, M.D.
Eric Feinstein, M.D.
Aisha Traish, M.D.
Case 2
AA is a 5-day-old infant female transferred to the neonatal ICU from an outside hospital for escalation of clinical care. You are consulted for further ophthalmic evaluation and management by the ICU team. Prenatal history is significant for poor prenatal care and alcohol abuse during pregnancy. She was born at 37 weeks without complications, but birth weight was noted to be in the third percentile ( Fig. 16.4a , b ).


Fig. 16.4
(a, b) External photograph of 5-day-old infant with lack of eyelids, eyelashes and eyebrows. There is significant injection and chemosis OU with bilateral infiltration and ulceration of the corneas OU. Multiple anomalies can be seen on the external photograph including sloping forehead, hypertelorism, flattened nasal bridge, micrognathia, macrostomia, deformed ears, short neck, hyperpigmentation and tense and loose skin in different areas. (c) External photograph showing significant thinning of the cornea. (d) External photograph after eyelid reconstruction and bilateral penetrating keratoplasty with clear grafts
On general examination, the patient has multiple, severe malformed features. Facial abnormalities include a sloped forehead, hypertelorism, flattened nasal bridge, macrostomia, micrognathia, deformed ear lobes, thin upper lip with long philtrum, and reduced hair of the scalp and eyebrows (Fig. 16.4a ). She also has absent nipples, abnormal skin tension throughout her entire body with absence of hair or lanugo, and abnormal genitalia.
On ophthalmic examination, she is found to be averse to light in either eye. There is full extraocular motility, and intraocular pressures measured by portable Tonopen are 10 mmHg in both eyes. On external exam, there is an absence of eyelids, eyelashes, and eyebrows with scant orbicularis muscle present with retraction of the globe when blinking (Fig. 16.4b ). There is marked conjunctival injection and chemosis bilaterally. The corneas are found to be opacified with infiltration and 60% thinning bilaterally and with associated corneal epithelial defects of 5 and 6 mm in the right and left eyes, respectively. Corneal diameters are 7 mm OD and 8 mm OS. There is no view to the anterior chamber, iris, or lens. B-scan ultrasonography does not reveal any posterior abnormalities.
What Differential Diagnoses Do These Findings Suggest? What Additional History Would This Exam Prompt You to Obtain?
This patient has bilateral cloudy corneas with ulceration, infiltration, and stromal loss. In this child with multiple other congenital abnormalities, an important piece of history to gather from the outside hospital records and physicians is whether the corneas were cloudy at birth or whether they became cloudy in the postnatal period. This would be the first major branch point for diagnostic consideration.
If the corneas were noted to be cloudy at birth, the differential diagnosis would include a distinct set of congenital causes. The most common reason for congenital clouding of the cornea is congenital glaucoma. Other major causes include: sclerocornea , tears in Descemet’s membrane (often caused by traumatic birth or forcep use), metabolic diseases such as Hurler Syndrome , Peters anomaly , congenital hereditary endothelial dystrophy (CHED) , congenital hereditary stromal dystrophy (CSHD) , and limbal dermoids. There have also been reports of children with fetal alcohol syndrome exhibiting bilateral diffusely cloudy corneas at birth, which would be an important consideration in this case given the mother’s alcohol abuse during pregnancy. Other rarer cause of congenital clouding of the cornea includes congenital rubella, posterior ulcer of von Hippel, posterior keratoconus, congenital corneal staphyloma, cornea plana, corneal keloids, oculoauriculovertebral dysplasia, posterior polymorphous dystrophy, and Fryns syndrome.
However, if the corneas were noted to be clear at birth with secondary clouding, then the differential diagnosis would heavily favor an infectious, inflammatory, or exposure-related etiology. In this case, the patient’s lack of eyelids predisposes the patient to developing severe exposure keratopathy. Infectious etiologies, including bacterial or fungal, cannot be ruled out in this case due to the patient’s extreme presentation and exposure to pathogens in the birth canal as well as in the hospital setting. Inflammatory etiologies are much less likely in a newborn as their immature immune systems are unlikely to produce such an exuberant response.
According to outside hospital records, the corneas were essentially clear at birth but have become increasingly opacified and thinned over the past few days, and concern for corneal perforation prompted the patient’s transfer to you.
What Is the Most Likely Diagnosis? What Additional Workup Would You Obtain for the Patient’s Ocular Findings?
The most likely diagnosis at this point given the constellation of findings is ablepharon macrostomia syndrome (AMS), which is on the spectrum of ectodermal dysplasias. AMS is an extremely rare disorder first described by McCarthy and West in 1977, which is characterized by the absence of eyelids, sparse or absence of hair, a large fish-like mouth (macrostomia), ear and genital anomalies, and redundant coarse skin [18–21]. As of 2011, fewer than 20 cases of AMS had been reported in the literature worldwide [18].
Patients with corneal ulceration should always be worked up and empirically treated for infectious etiologies until it has been ruled out. A panculture including aerobic, anaerobic, fungal, and acid-fast cultures should be obtained. In neonates, this can be done most efficiently with topical anesthetic, calcium alginate swabs, and the aid of a portable slit lamp, if available, while an assistant holds the patient’s head still; if the neonate can tolerate this bedside intervention, one can avoid unnecessary exposure to general anesthesia.
Aerobic, anaerobic, fungal, and acid-fast Bacillus cultures of the cornea are negative with no organisms seen on gram stain.
What are Your Next Steps in Management?
Even before knowing the culture results, the most emergent issue in this case is to stop the cycle of continued corneal ulceration and stromal loss, which is rapidly heading toward corneal perforation. The importance of diagnosing ablepharon macrostomia syndrome is to institute early treatment, starting with conservative measures such as lubrication with artificial lubricant tears, ointments, moisture chambers, etc. Ideally, these measures should be instituted as soon as the child is born and the anomalies are noted, in order to prevent progression of disease.
In cases where aggressive lubrication is inadequate or where the condition has already progressed to a severe stage upon presentation, as in our case, conservative therapies may be insufficient. There have been reports about AMS discussing the use of tarsorrhaphies [22], but these are of limited use in the severest cases where there is a complete absence of eyelids. One case report of a patient with severe exposure keratopathy and corneal desiccation due to congenital cryptophthalmos [23] described the use of an amniotic membrane to line the ocular surface and prevent further exposure. Without eyelids and associated meibomian glands, the sequelae of severe exposure keratopathy present rapidly; therefore it is important to stage treatment with both temporary (eye lubricants and amniotic membrane) and permanent solutions (eyelid reconstruction and tectonic corneal stability) to maximize visual outcomes.
Cryopreserved amniotic membranes were placed across both corneas at the bedside.
What Additional Systemic Workup Would You Recommend?
An important aspect of management for these patients is to obtain a full systemic workup in order to evaluate for life-threatening birth defects, such as congenital heart anomalies. Although cardiac anomalies are not part of the constellation of findings in ablepharon macrostomia syndrome, failure to diagnose cardiac anomalies may lead to worse overall outcomes. The management of these patients is also best approached in a team-based format, in conjunction with neonatal intensivists, pediatric otolaryngologists, and geneticists.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


