Fig. 23.1
Left inferior parathyroid adenoma abutting left common carotid artery and dorsal to left thyroid lobe. Transverse view. CCA common carotid artery, IJV internal jugular vein, SH/ST sternohyoid and sternothyroid muscles, PTA parathyroid adenoma, SCM sternocleidomastoid
In patients suspected of having secondary or tertiary hyperparathyroidism, multigland hyperplasia is more commonly encountered than a solitary adenoma. Four-gland hyperplasia can usually be appreciated on ultrasound. In parathyroid hyperplasia , the four glands may not be uniformly enlarged, as one or more glands may demonstrate greater hyperplastic changes than the others [7–9] (Fig. 23.2). Ultrasound assessment may aid in planning the order of exploration, from the most prominently enlarged gland downwards, while following intraoperative PTH levels. Again thorough assessment of the thyroid gland will be important for planning the appropriate operation [10].


Fig. 23.2
Parathyroid hyperplasia showing a larger superior parathyroid and smaller inferior parathyroid gland. Both glands are hyperplastic but are asymmetrically enlarged. Sagittal view. SPT superior parathyroid, IPT inferior parathyroid, SH/ST sternohyoid and sternothyroid muscles
Neither radionuclide scanning nor parathyroid ultrasound exhibit absolute sensitivity nor specificity for parathyroid adenomas. A strongly localizing result from one image test may be sufficient to direct surgery, but it does not preclude an indication for radionuclide scanning or other imaging modalities [11, 12]. Identification of a cervical parathyroid adenoma candidate on ultrasound does not rule out the possibility of a second, ectopic adenoma in a location inaccessible to ultrasound (e.g., mediastinum, retropharynx). Whereas an ultrasound may reveal a hypoechoic lesion in an anatomically appropriate location for a parathyroid gland, a small lymph node may be difficult to distinguish from parathyroid tissue sonographically (Fig. 23.3). Sestamibi radionuclide scanning may help scrutinize a parathyroid candidate further with a functional evaluation. Conversely, a “positive” sestamibi radionuclide scan that suggests a sidedness to a parathyroid candidate is ideally supported by an ultrasound that demonstrates an enlarged hypoechoic lesion with the same laterality, as radionuclide scan interpretation is subject to distraction by background noise inherent to the properties of this imaging technique.


Fig. 23.3
Left level 6 lymph node . Transverse (left) and sagittal (right) views. LN lymph node
Preoperative or intraoperative ultrasound of the neck allows the surgeon to identify aberrant anatomy prior to the operation. For example a non-recurrent right laryngeal nerve is readily suspected if the right subclavian and carotid arteries do not proximally arise from an innominate artery, which should be identifiable on ultrasound in most patients [13]. While it is not necessary in all parathyroid cases to identify the recurrent (or non-recurrent) laryngeal nerve, its anatomical course should be understood and anticipated as well as possible in any central neck compartment surgery (Fig. 23.4 and Video 23.1).


Fig. 23.4
Right common carotid and subclavian artery confluence. CCA common carotid artery, SCA subclavian artery, INA innominate artery
Ultrasonographic Appearance of Parathyroid Tissue
Normal parathyroid glands are typically less than 40 mg in mass, and measure approximately 6 mm in cranio-caudad length, and 3–4 mm in width [3, 14]. They are typically too small and too similar to surrounding structures to be appreciated by ultrasound imaging. In the majority of cases, ultrasound imaging of pathologically enlarged parathyroid glands demonstrates a homogeneous ovoid, hypoechoic lesion that may have a distinct, more echogenic rim [15–17]. Less commonly, enlarged parathyroid glands may be multilobulated in shape; they may also appear anechoic, or even show echogenicity similar to thyroid tissue. The appearance may be difficult to distinguish from a lymph node, which also may be ovoid with a hypoechoic appearance. In enlarged lymph nodes, a fatty central hilum may be visible as a more echogenic region, and Doppler signaling for vascular flow should demonstrate central flow through the hilar vascular pedicle. In contrast, a parathyroid adenoma has a primarily polar vascular pedicle—rather than central within the ovoid mass; the pedicle typically terminates at the superior pole (Fig. 23.5 and Videos 23.2, 23.3, and 23.4). This may be best appreciated in sagittal orientation on ultrasound. A vascular “arc” has also been described coursing over the surface of parathyroid tissue [18]. Hypervascularity of the parathyroid capsule, or hypervascularity of the adjacent thyroid gland, has also been reported on ultrasound Doppler flow studies [19]. The contrasting vascular characteristics of lymph nodes and parathyroid adenomas are difficult to discern in small lymph nodes, in which ultrasonographic resolution is insufficient to visualize the central hilum clearly.
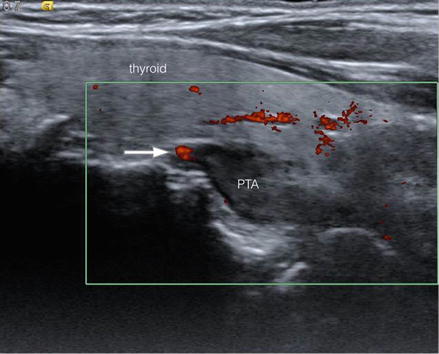

Fig. 23.5
Left inferior parathyroid adenoma showing superior polar vascularity (arrow). Sagittal view. PTA parathyroid adenoma
A parathyroid candidate on ultrasound may also be difficult to distinguish from a thyroid nodule if an intracapsular parathyroid gland is suspected (Fig. 23.6). While echogenicity of the lesion may be somewhat helpful, many thyroid nodules are hypoechoic, round, or ovoid in appearance. Ordinarily a subcentimeter thyroid nodule without suspicious characteristics (e.g., microcalcification, irregular borders) may not warrant a fine-needle aspiration (FNA) biopsy . However if such a nodule is a candidate for parathyroid tissue, an ultrasound-guided FNA biopsy with saline washings for parathyroid hormone (PTH) levels is a useful next diagnostic step, provided that accurate aspiration of the lesion is achieved [20, 21].


Fig. 23.6
Right intracapsular parathyroid adenoma. Transverse (left) and sagittal (right) views. PTA parathyroid adenoma, CCA common carotid artery, IJV internal jugular vein
Patients diagnosed with primary hyperparathyroidism will be found to have a solitary adenoma in 85 % of cases; multigland disease, either diffuse hyperplasia or less commonly double adenomas in 15 % of cases; and in less than 1 % of cases, parathyroid carcinoma [3]. The key distinguishing factor between the rare parathyroid carcinoma and an adenoma on ultrasound is the invasion, by carcinoma, into adjacent structures that may be visualized on ultrasound, with an irregular lesion border. Physical exam findings of a palpable mass, and laboratory findings of a serum calcium greater than 14 mg/dL, a PTH greater than three times normal, and a markedly elevated serum alkaline phosphatase should also increase suspicion for parathyroid malignancy, although these each has low specificity [22, 23]. In multiple endocrine neoplasia types I and IIa, as well as secondary and tertiary hyperparathyroidism, multigland hyperplasia is typically appreciated on ultrasound, whereas sporadic cases of multiglandular disease may not present with enlarged parathyroid glands to a size visible on ultrasound.
Embryology and Location of the Parathyroid Glands
A brief review of the embryologic development of the parathyroid glands is useful to aid in understanding their most common anatomical locations and methodically survey potential ectopic sites for ultrasonographic identification. The superior and inferior parathyroid glands develop from the dorsal wings of the embryonic fourth and third pharyngeal pouches, respectively. Differentiation into parathyroid parenchyma occurs during the fifth to sixth week of gestation, followed by detachment from the pharynx at week 7 after which both pairs of glands begin a caudal migration. The superior parathyroid glands attach to the thyroid and migrate caudally for a much shorter distance than the inferior parathyroid glands. The superior parathyroid glands typically deposit along the posterior aspect of the mid to superior thyroid lobe. Whereas the inferior parathyroid glands develop from the dorsal wing of the third pharyngeal pouch, the thymus develops from the ventral wing of this same pouch. As the thymus migrates caudally into the superior mediastinum, the attached inferior parathyroid glands follow; however they usually detach from the thymus and deposit proximally, just inferior and posterior to the inferior thyroid pole.
The superior parathyroid gland is located posterior, or dorsal, to the recurrent laryngeal nerve (RLN) , whereas the inferior parathyroid rests anterior, or ventral, to the nerve. Since the RLN is not visualized on parathyroid ultrasound, the posterior, or deep, border of the carotid artery or the inferior thyroid artery serves as sonographic surrogate landmark for the plane of the RLN (Figs. 23.7 and 23.8). For example a parathyroid candidate lying anterior, or superficial, to this plane would likely be an inferior parathyroid.



Fig. 23.7
A descended left superior parathyroid adenoma, abutting esophagus deep to posterior plane of the common carotid artery. Intraoperatively, the recurrent laryngeal nerve was identified ventral to the adenoma, as expected in relation to a superior parathyroid gland. Transverse view. PTA parathyroid adenoma, CCA common carotid artery, EGS esophagus

Fig. 23.8
Left superior parathyroid adenoma. Transverse (left) and sagittal (right) views. PTA parathyroid adenoma, CCA common carotid artery, SCM sternocleidomastoid, SH/ST sternohyoid and sternothyroid muscles
During a parathyroid ultrasound survey , it is helpful to anticipate typical and ectopic locations of parathyroid glands based on their embryological development. The superior parathyroid glands may be identified as high as the hyoid bone or level of the carotid bifurcation. In addition to the central compartment proper, ultrasound should examine the carotid sheath, retropharyngeal, retroesophageal, retrotracheal, and parapharyngeal spaces to the extent possible for potential ectopic glands [24]. The superior parathyroid gland may also descend into the posterior mediastinum, a space bounded superiorly by the upper border of pericardium, and inferiorly by the diaphragm, beyond visibility by transcutaneous ultrasound . The inferior parathyroid gland may continue to descend into the superior, or even anterior (anteroinferior) mediastinum as it follows the descent of the thymus. The inferior parathyroid gland may be found as far cephalic as the hyoid bone as well if it failed to descend with the thymus. A parathyroid candidate at this cephalic limit would be considered inferior or superior based on its relationship to the RLN.
Techniques in Parathyroid Ultrasound
Ultrasound evaluation of parathyroid disease should first begin with a thorough survey of the central neck compartment , from hyoid bone cephalically to the thoracic inlet as far caudally as can be accessed by the ultrasound transducer [18]. A suggested approach begins by identification of the innominate artery as it courses from the superior mediastinum towards the right central neck compartment, branching into the right common carotid and subclavian artery. The space from right carotid sheath to larynx and trachea is carefully examined in a caudal to cephalad direction with the transducer in a transverse orientation. Examination continues to the superior limit of the thyroid lobe, and can extend to the hyoid. Any parathyroid candidates are then investigated with the transducer in a sagittal plane, which aids in visualizing the vascular pedicle that frequently can be seen at the superior pole of the enlarged gland. Examining the right central neck compartment beginning at the right innominate artery also provides rapid confirmation of the normal great vessel anatomy, and therefore RLN anatomy. The awake patient can be asked to turn his or her head towards and away from the examiner to further enhance visualization of otherwise overlapping structures; in the sedated patient, the examiner may need to carefully rotate the patient’s head to accomplish the same result. An awake patient can also be asked to swallow, which can aid in distinguishing the esophagus from other nodular structures. The anesthetized patient may have an endotracheal tube and/or an esophageal probe in place that can distort anatomy or create ultrasound artifacts, such as acoustic shadowing, and should be noted.
The left central neck compartment is subsequently evaluated from the thoracic inlet to superior limit of the thyroid lobe, again from carotid sheath laterally to the trachea and larynx medially. Both transverse and sagittal transducer orientations are employed. Next the thyroid gland itself is evaluated, beginning with the right thyroid lobe from caudal to cephalad, transversely, and then in sagittal view. This step is repeated on the left, followed by examining the isthmus. As discussed earlier, it is worthwhile to perform thyroid ultrasound in all hyperparathyroidism patients, both in search of ectopic intrathyroidal parathyroid glands and to assess possible thyroid pathology that may warrant further diagnostic and therapeutic measures. Ultrasound-guided FNA biopsy of suspicious intrathyroidal lesions with saline needle washing for PTH assay can then be employed [21]. Often the middle thyroid vein is seen draining from the mid to lower thyroid lobe, and is a useful surgical landmark that may help orient the surgeon if a parathyroid candidate is visualized nearby (Fig. 23.9).




