18.1 Features
Diabetic retinopathy (DR) represents a retinal microvascular complication of diabetes mellitus (DM) and is the foremost cause of preventable vision loss in working age adults; its prevalence has been estimated at nearly 35% of all diabetic adults aged 40 and over. DR is more common and severe in patients with long-standing or poorly controlled diabetes mellitus. Early detection of retinopathy in diabetic patients is critical since the first stages are asymptomatic. The risk of developing DR increases with patient age and the duration of diabetes. Control of blood glucose, blood pressure, and blood lipids are also modifiable risk factors. Hemoglobin A1c level in most patients should be 7% or lower. Diabetic eye disease can lead to visual loss through a variety of mechanisms, including DME, macular ischemia, vitreous hemorrhage, and traction retinal detachment.
The clinical presentation of DR subdivide it into two stages: (1) nonproliferative diabetic retinopathy (NPDR), which represents microvascular abnormalities without neovascularization/fibrovascular tissue and (2) proliferative diabetic retinopathy (PDR), the most advanced stage of DR characterized by the development neovascularization. Each of these two stages can also be subdivided into various levels of severity (e.g., mild, moderate, severe for NPDR; ▶ Fig. 18.1, ▶ Fig. 18.2, ▶ Fig. 18.3). PDR may also be described by the presence or absence of high-risk features (e.g., vitreous hemorrhage, size/location of neovascularization, traction retinal detachment; ▶ Fig. 18.4, ▶ Fig. 18.5). DME is classified separately from the stages of DR as it can be found in both nonproliferative and proliferative groups (▶ Fig. 18.6, ▶ Fig. 18.7). DME represents the breakdown of the inner blood/retinal barrier leading to capillary leakage and macular swelling.

Fig. 18.1 Fundus photograph of moderate nonproliferative diabetic retinopathy.

Fig. 18.2 Fundus photograph montage of severe nonproliferative diabetic retinopathy with microaneurysms, intraretinal hemorrhages, cotton wool spots, and venous beading of arcade vessels.

Fig. 18.3 Fundus photograph of moderate nonproliferative diabetic retinopathy associated with diabetic macular edema demonstrating a circinate ring of hard exudates surrounding a group of microaneurysms.

Fig. 18.4 (a,b) Fundus photograph of proliferative diabetic retinopathy demonstrating neovascularization of the disc.

Fig. 18.5 Fundus photograph of severe proliferative diabetic retinopathy with associated fibrovascular proliferation and extensive traction retinal detachment.

Fig. 18.6 Optical coherence tomography of diabetic macular edema, note hard exudates located mainly in the outer plexiform and some in the outer nuclear layer of the retina.

Fig. 18.7 (a) Optical coherence tomography of severe diabetic macular edema with extensive intraretinal fluid and subretinal fluid. (b) Following anti-vascular endothelial growth factor therapy, dramatic improvement with near resolution of fluid with residual ellipsoid zone attenuation and dissociated inner retinal layers.
18.1.1 Common Symptoms
Asymptomatic in early stages. Symptoms can vary widely based on the disease presentation, but may include floaters (e.g., vitreous hemorrhage), vision loss (e.g., DME, traction retinal detachment, vitreous hemorrhage), and metamorphopsia (e.g., DME). Symptoms usually affect both eyes asymmetrically.
18.1.2 Exam Findings
Nonproliferative Diabetic Retinopathy
Clinically, microaneurysms are often the earliest manifestation of NPDR. Other classic retinal lesions include intraretinal hemorrhages, hard exudates, cotton wool spots, intraretinal microvascular abnormalities (IRMA, i.e., dilated capillaries between an arteriole and venule), and venous beading (i.e., alternating areas of venous dilation and constriction). As defined by the Early Treatment Diabetic Retinopathy Study (ETDRS), severe NPDR is characterized by any of the following (4:2:1 rule): intraretinal hemorrhages in four quadrants, venous beading in two quadrants, or IRMA in one quadrant (▶ Fig. 18.2).
Diabetic Macular Edema
DME is represented by intraretinal fluid accumulation and macular thickening. It may be defined as focal or diffuse macular edema based on the leakage pattern; focal macular edema is caused by foci of capillary abnormalities such as microaneurysms usually associated with a circinate ring of hard exudates (▶ Fig. 18.3); diffuse macular edema is caused by extensive leakage and may be associated with large cystoid spaces. Currently, DME is also described based on location on optical coherence tomography (OCT), including noncentral DME or central-involving DME. Historically, clinically significant macular edema (CSME) is defined when one of the following conditions occur: retinal thickening at or within 500 µm of the center of the macula, hard exudate at or within 500 µm of the center of the macula with adjacent thickening, or retinal thickening larger than 1 disc area located within 1 disc diameter of the center of the macula (▶ Fig. 18.3, ▶ Fig. 18.6, ▶ Fig. 18.7). However, with the advent of OCT, the clinical entity of CSME is less commonly used as a treatment criteria compared to DME location.
Proliferative Diabetic Retinopathy
PDR is characterized by the onset of neovascularization resulting from retinal ischemia. The new vessels, located at the disc (NVD) or elsewhere in the retina (NVE), may cause preretinal and vitreous hemorrhages (▶ Fig. 18.4). Neovascularization may also fibrose and contract (fibrovascular proliferation) resulting in epiretinal membrane formation, vitreoretinal traction and/or traction retinal detachments (TRD; ▶ Fig. 18.5). According to the Diabetic Retinopathy Study (DRS), high-risk PDR is defined when NVD is accompanied by vitreous hemorrhage, when NVD occupies greater than one-quarter to one-third disc area, or when NVE is larger than one-half disc area with vitreous hemorrhage.
18.2 Key Diagnostic Tests and Findings
18.2.1 Optical Coherence Tomography
Gold standard for evaluating for the presence of macular edema and vitreoretinal interface abnormalities. Allows for quantitative retinal thickness analysis and is the most common method for evaluating need for treatment for DME and assessing treatment response to therapy (▶ Fig. 18.6, ▶ Fig. 18.7).
18.2.2 Fluorescein Angiography
Fluorescein angiography (FA) provides an important evaluation for retinal vascular dynamics, including microaneurysms, leakage, nonperfusion, and neovascularization. Ultra-widefield fluorescein angiography (UWFA) provides panretinal assessment of retinal vascular abnormalities and enables greater visualization of the retinal periphery compared to conventional FA (▶ Fig. 18.8). UWFA appears to be more sensitive for detection of neovascularization and other peripheral lesions. FA may be used as a guide for targeting laser photocoagulation, both for focal laser treatment and panretinal photocoagulation (▶ Fig. 18.9).
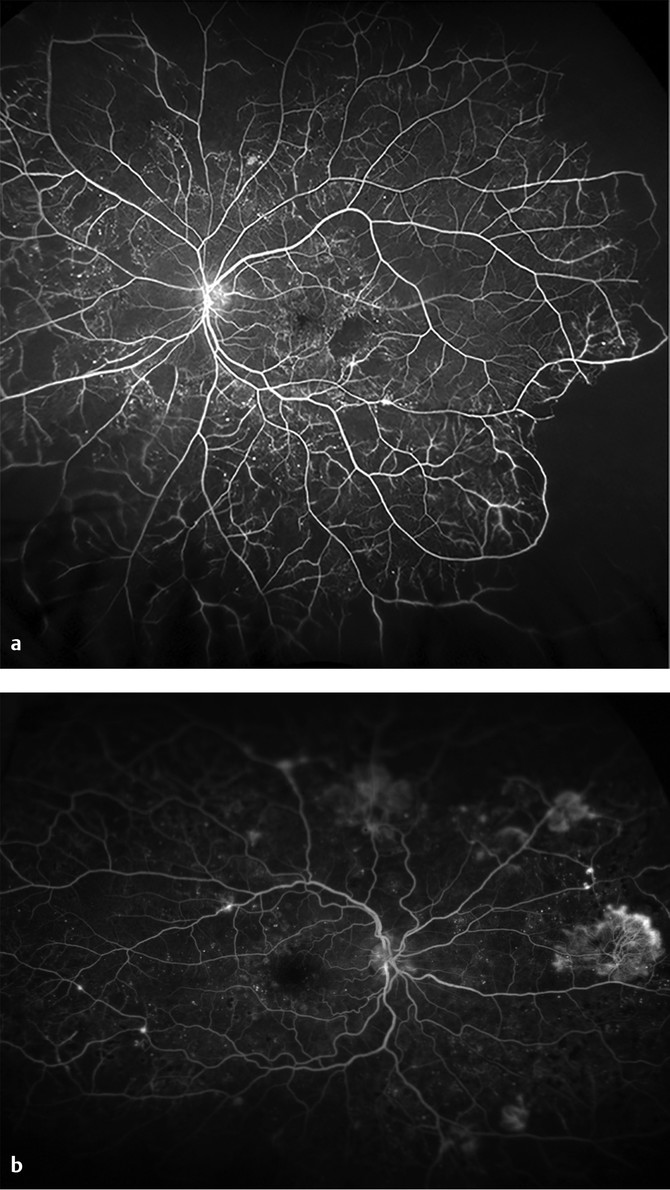
Fig. 18.8 (a) Ultra-widefield fluorescein angiography (UWFA) with proliferative diabetic retinopathy (PDR) showing microaneurysms and severe peripheral nonperfusion with areas of vascular leakage and minimal neovascularization. (b) UWFA in PDR demonstrating extensive neovascularization throughout the retinal periphery, nonperfusion, microaneurysms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


