Fig. 5.1
Simplified representation of the classic hypothalamic–pituitary–adrenal (HPA) axis. ACTH adrenocorticotropic hormone, CRF corticotropin-releasing factor. Redrawn and simplified from Basappa et al. (2012)
The HPA axis involves adaptation to increased demands and maintains homeostasis after stressful challenges, but it also supports normal physiology and homeostasis (Canlon et al. 2007). The overall function of the HPA axis is controlled by several negative feedback loops (Fig. 5.1). A dysfunctional HPA axis is associated with manifestations of psychosomatic and psychiatric disorders. Hyperactivity of the HPA axis is often found in major depression and is associated with increased susceptibility to infection and cardiovascular problems (McEwen 2007). The glucocorticoid receptors, affecting the main targets of the HPA axis, are important regulators for protecting against noise trauma (Canlon et al. 2007).
5.3 Recognizing Stress in Animals
Stress does not occur unless the animal perceives a threat (Carstens and Moberg 2000). Because most stressors are brief, the changes in biological function required to cope with the stressor are minimal and of little consequence to the animal’s well-being (Carstens and Moberg 2000). These brief stressors include stressors associated with the experimental manipulation and handling of the animal (Carstens and Moberg 2000). According to Bali and Jaggi (2015), stress in animals may be assessed (1) at the behavioral level reflected in social interaction, (2) at the biochemical level by measuring plasma corticosterone and ACTH, and (3) at the physiological level by measuring food intake and body weight. Carstens and Moberg (2000) pointed out that the stressor responses of the HPA axis provide an example of the difficulty encountered in measuring stress: “Measuring the secretion of the glucocorticosteroids—cortisol (primates) and corticosterone (rodents)—has been the most popular tool for evaluating stress, and frequently increases in circulating glucocorticosteroids have been used as proof of stress. It is evident that numerous stressors do elicit an increase in circulating steroids but not all stressors elicit an HPA response” (Carstens and Moberg 2000).
5.4 Causing Stress in Animals
The well-described stress models used in research include immobilization, restraint, electric foot shock, and social isolation (Bali and Jaggi 2015). We will first describe restraint stress and foot-shock stress, as they are most used in auditory research. However, we should also realize that noise exposure in itself is a stressor associated with increase in plasma norepinephrine levels in awake animals (Muchnik et al. 1998).
5.4.1 Restraint Stress
Restraint stress is a form of immobilization stress in which animals are not allowed to move for a specified period of time. Restraint stress is induced by placing the test animal in a plastic tube or wire-mesh container. This does allow limb movement but limits the range of overall movement. Based on neural and endocrine responses, restraint stress appears to be less intense than immobilization. Restraint stress is a commonly employed model for the induction of acute as well as chronic stress in rodents (Bali and Jaggi 2015).
5.4.2 Electric Foot Shock-Induced Stress
The electric foot shock paradigm mainly comprises acute or chronic exposures of foot shocks with variable intensity and duration on an electrified grid floor in an electric foot shock apparatus. Electric foot shocks are an integral part of classical conditioning tests used to assess the presence of tinnitus in animals (Sect. 5.8). It is generally not appreciated that foot shocks cause acute stress and may affect the very thing the procedure aims to measure. As Bali and Jaggi (2015) noted: “Electric foot shock stressor includes both physical as well as emotional components and it is used as direct (physical stress) and indirect stressor (psychological stress). It has been mainly used with varying degree to produce mild as well severe stress of both acute and chronic in nature.”
5.4.3 Noise-Induced Stress
Chronic noise-induced activation of the HPA axis might cause a variety of problems because of abnormally high levels of circulating stress hormones. The auditory system connects via the amygdala and other circuits to the HPA axis and can thereby cause the release of stress-related hormones (Fig. 5.2). Henkin and Knigge (1963) suggested that noise-induced corticosterone elevations persist for up to ~12 h after stress induction. Noise-induced stress can affect arousal and startle responses, the latter being more and more used in the gap-startle test for tinnitus in animals. We will expand on this in Sect. 5.8.
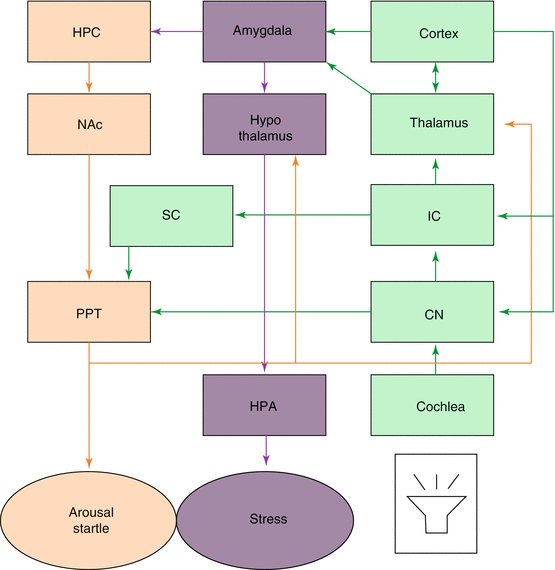

Fig. 5.2
Schematic of the various interconnections between the auditory system and the structures involved in noise-induced arousal, startle, and stress. The green boxes and lines represent the auditory system. The purple boxes and lines the HPA stress system. The orange boxes and lines represent the arousal and startle system. CN cochlear nucleus, IC inferior colliculus, SC superior colliculus, HPC hippocampus, NAc nucleus acumbens, PPT pedunculopontine tegmental nucleus, HPA hypothalamic-pituitary-adrenal axis. Modified from Eggermont (2013a)
The mechanism of noise-induced stress on the cochlea briefly can be described as (Eggermont 2013a): noise exposure activates neuroendocrine cells containing corticotropin-releasing hormone (CRH) in the hypothalamic paraventricular nucleus, which stimulates the release of ACTH in the pituitary gland (Fig. 5.1). ACTH release and the resulting secretion of corticosterone (in rodents) in the adrenal gland increase with noise intensity. The increased levels of ACTH as well as corticosterone remained elevated for the duration of noise presentation along with behavioral stress response. As we have seen, corticosterone in turn activates glucocorticoid receptors in target structures such as the inner ear (Kraus and Canlon 2012).
5.5 Stress and the Cochlea
5.5.1 The HPA Axis Signaling System
The effects of noise stress on the cochlea are well studied (Horner 2003). After exposing rats daily to 85 dB SPL white noise for 4 h on 3 consecutive days, Rarey et al. (1995) detected a significant decrease in glucocorticoid receptor protein levels in the organ of Corti, but not in the spiral ligament, together with a significant increase in serum corticosterone levels compared to nonexposed controls. Curtis and Rarey (1995) then used immobilization stress. They observed a significant quadratic trend of GR levels in spiral ligament tissues of rats restrained from 6 h daily. GR levels were elevated by day 2, and by day 21 GR levels had returned to near normal values. There was also a statistically significant decrease in the organ of Corti’s GR levels when the daily restraint stress was applied for up to 7 days, but was again no longer observed after 21 days.
There are several reports demonstrating that acute stress can protect the auditory system. Noise-induced temporary threshold shifts after noise exposure (120 dB, 20 min) were less in stressed guinea pigs than in unstressed controls (Muchnik et al., 1998). Wang and Liberman (2002) showed that two 12-h epochs of mild physical restraint significantly reduced permanent threshold shifts from a subsequent acoustic overexposure, as long as the treatment-trauma interval was short (≤2 h). The period of protection coincided with the period of elevated corticosterone levels. Thus, cochlear protective effects of sound conditioning may be mediated by stress pathways through the activation of glucocorticoid receptors in the inner ear (Canlon et al. 2007; Meltser and Canlon 2011). Kraus and Canlon (2012) summarized this as: “while there are positive correlations between stress and hearing problems, a large body of studies provides evidence that acute stress can enhance hearing or mediate protection against noise-induced hearing loss.”
Mazurek et al. (2010) examined the effect of stress on the auditory system of Wistar rats. Stress was induced by a combination of handling the animals, moving the animals to a new cage and a different room, exposed them to unpleasant sound and vibration, and restrain them. The unstressed control animals were kept in their home cage. Mazurek et al. (2010) found that such induced 24-h stress decreased auditory brainstem response (ABR) thresholds and increased ABR amplitude and strength of distortion product otoacoustic emissions (DPOAEs). The increased ABR and DPOAE amplitudes were most pronounced between 3 and 6 h post-stress, and 1 week later returned to control levels. Corticosterone and tumor necrosis factor alpha concentrations were systemically elevated in stressed animals between 3 and 6 h post-stress, pointing to the activation of the HPA axis. Expression of the HPA-axis-associated GR and hypoxia-inducible factor 1 alpha (Hif1a) genes was modulated in some auditory tissues. In the inferior colliculus (IC), Mazurek et al. (2010) found an upregulation of GR mRNA 3 h post-stress and continuous upregulation of Hif1a up to 24 h post-stress. In the spiral ganglion, there were no differences in gene expression between stressed and control animals. In the organ of Corti, no changes in the expression of GR mRNA were found; however, the expression of Hif1a was significantly downregulated 1 week after stress induction. In addition, the expression of prestin in the OHCs was significantly upregulated 6 h post-stress. Mazurek et al. (2010) concluded “that 24-h stress induces transient hypersensitivity of the auditory system and modulates gene expression in a tissue-specific manner.” Knipper et al. (2013) reported an influence of stress on the IHC synapse in rodents. Two days after stress induction, the number of release sites (ribbons) at IHC synapses was increased in animals that exhibited high corticosterone levels (Singer et al. 2013).
5.5.2 The Local Cochlear CRF-Signaling System
Vetter and colleagues (Basappa et al. 2012) recently discovered “a novel cochlear signaling system that is molecularly equivalent to the classic hypothalamic–pituitary–adrenal (HPA) axis.” This cochlear signaling system balances auditory sensitivity and susceptibility to noise-induced hearing loss and protects against metabolic insults from exposures to ototoxic drugs (Basappa et al. 2012). This local HPA system appears independent of the systemic HPA signaling to the cochlea. It consists of locally produced CRF, a CRF1-receptor, and ACTH (Fig. 5.3). Deletion of the CRF1-receptor gene resulted in auditory impairment of knockout animals (Graham and Vetter 2011). As we have seen, systemic HPA activation also influences hearing via delivery of systemic glucocorticoids through the circulation (Basappa et al. 2012).


Fig. 5.3
The cochlea CRF signaling system (top) expresses an HPA-equivalent signaling system (bottom). ACTH adrenocorticothropin, CRF corticotropin-releasing factor, CRF1 and CRF2 are CRF-receptors, MC2R mineralcorticoid receptor, POMC pro-opiomelanocortin. From Basappa et al. (2012)
5.6 Stress and the Central Nervous System
Mazurek et al. (2015) emphasized that stress causes changes in neuroplasticity. Auditory neural plasticity may be defined as the dynamic changes that occur in the structural and functional characteristics of auditory neurons in response to changes in, or in the significance of, the sound they receive (Irvine 2010). Synaptic plasticity affecting the glutamate postsynaptic system and especially the AMPA and NMDA receptors appears to be regulated by stress (Hubert et al. 2014; Timmermans et al. 2013). A stressful acoustic stimulus, such as noise, causes amygdala-mediated release of stress hormones via the HPA-axis, which may have negative effects on the central nervous system (Fig. 5.2). The hippocampus can affect auditory processing by being able to mediate novelty detection. Noise exposure affects hippocampal neurogenesis and LTP in a manner that affects structural plasticity, learning, and memory (Kraus and Canlon 2012). High stress levels at the time of a moderate auditory trauma led to a “tinnitus-specific” central responsiveness, including more severe IHC ribbon loss, reduction of ABR amplitudes, and the decline of Arc/Arg3.1 expression levels in the hippocampal CA1 or auditory cortex (Singer et al. 2013). In contrast, moderate stress levels at the time of trauma could prevent such a tinnitus-specific central response and restore adaptive central responses (Knipper et al. 2013).
Nava et al. (2017) studied the time course of acute stress by foot shock on dendritic remodeling within the prelimbic (PL) region of the rodent prefrontal cortex (PFC). They analyzed dendritic length and spine density at 1 day, 7 days, and 14 days after inducing stress. At day 1, they found increased small-spine density and dendritic retraction, together with significant atrophy of apical dendrites. After 7 and 14 days recovery, complete normalization of spine density was observed. Nava et al. (2017) concluded that acute stressors may induce rapid and sustained changes of PL neurons. These changes in the PFC could affect the protective gating effect that was hypothesized to prevent tinnitus (Leaver et al. 2011; next section).
5.7 Stress and Tinnitus
Tinnitus is strongly associated with emotional stress, anxiety, and depression (Langguth 2011; Mazurek et al. 2012). Like external environmental noise, the internally generated noise of tinnitus may cause emotional distress resulting in mood disorders like depression. In turn, stress or depression may contribute to the development of tinnitus (Halford and Anderson 1991; Robinson et al. 2007; Canlon et al. 2013). Reciprocal interactions of auditory areas and areas processing emotion seem essential for tinnitus generation (Rauschecker et al. 2010; Langguth et al. 2011; Fig. 5.4). The phantom sound may be caused by disinhibition, increased spontaneous firing rates, increased neural synchronization, and tonotopic reorganization in the central auditory system (Eggermont and Roberts 2004; Roberts et al. 2010). Furthermore, since the auditory and limbic systems are interconnected, tinnitus can affect emotional as well as cognitive properties of the limbic system. In turn, the limbic system may play a role for tinnitus generation or stabilization.
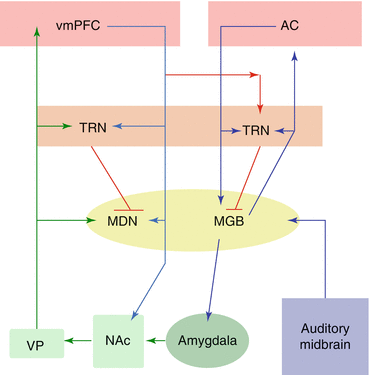

Fig. 5.4
Schematic of putative auditory-limbic interactions in tinnitus. Sensory input originates from the auditory midbrain (purple) and enters both auditory and limbic circuits via the medial geniculate body (MGB; yellow). Under normal circumstances, the limbic system may identify a sensory signal as perceptually irrelevant and inhibit the unwanted signal at the MGB via projections from the ventromedial prefrontal cortex (vmPFC; pink) to the auditory thalamic reticular nucleus (TRN) via the red pathway. In chronic tinnitus, inefficient vmPFC output via the red pathway prevents inhibition of the tinnitus signal at the level of the MGB, resulting in a constant perceptual presence of the tinnitus signal. Cortical structures are indicated in pink, the thalamus is indicated in yellow, the thalamic reticular nucleus in orange, the basal ganglia in light green, and the amygdala in dark green. MDN medial dorsal nucleus, VP ventral pallidum, AC auditory cortex, NAc nucleus accumbens. Based on Leaver et al. (2011)
Canlon et al. (2013) described findings in a cross-sectional study on the association of hyperacusis and stress on tinnitus, assessed by the tinnitus handicap questionnaire (THQ; Kuk et al. 1990) score. They found that the only significant predictors of mean THQ score for the left ear were hyperacusis and stress and only stress for the right ear. Canlon et al. (2013) suggested that stress seems an important predictor of tinnitus severity.
5.7.1 Stress Causing Tinnitus
Hu et al. (2010) used a mild-chronic stress model of depression in Sprague-Dawley rat and evaluated the effects with positron emission tomography (PET) imaging technique. They used both physical stressors (e.g., sleep deprivation, water deprivation, and heat stress) and psychosocial stressors (e.g., crowding, loud sound). After 4 weeks of random mixed stressing, brain PET analysis showed activation of left auditory cortex and deactivation of left inferior colliculus. No changes were detectable in the visual pathway. Changes in the auditory system correlated significantly with the depressive symptoms of experimental animals (Hu et al. 2010). Although this does not directly relates to tinnitus, the comorbidity with depressive symptoms and increased metabolic activation in auditory cortex is suggestive.
Another link to tinnitus may be found in the stress-induced increase in central opioid-dynorphin activity that potentiates the HPA-axis. Opioid peptides are found within and released from neural systems that regulate the body’s overall biologic response to physical/emotional stress. Emotional or physical stress induces potent analgesic effects, and the biologic response to stress is likely to involve multiple opioid systems (Sahley et al. 2013). Naturally occurring opioid dynorphins are also released from lateral efferent olivocochlear axons into the synaptic region beneath the cochlear IHCs during stressful episodes (Sahley and Nodar 2001). This results in altered neural excitability and/or distribution of spontaneous firing rates in type I auditory nerve fibers with low SFRs and high thresholds. Incidentally, the same subset of type I nerve fibers is affected by TTS, via the damaged ribbon synapses (Kujawa and Liberman 2009). Sahley et al. (2013) wondered if a lateral efferent olivocochlear-activated release of endogenous dynorphins may generate increased SFRs in ANFs (in the same way as induced by salicylate; Ruel et al. 2008) that could be processed by the central auditory system as either an acute subjective tinnitus. This so far remains in the domain of speculation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


