Purpose
The aim of this study was to investigate the safety and performance of the second generation of an implantable intraocular pressure (IOP) sensor in patients with primary open angle glaucoma (POAG).
Design
prospective, noncomparative, open-label, multicenter clinical investigation.
Methods
In this study, patients with POAG, regularly scheduled for cataract surgery, were implanted with a ring-shaped, sulcus-placed, foldable IOP sensor in a single procedure after intraocular lens implantation. Surgical complications as well as adverse events (AEs) during 12 months of follow-up were recorded. At each follow-up visit, a complete ophthalmic examination, including visual acuity, IOP, slit lamp examination, and dilated funduscopy as well as comparative measurements between Goldmann applanation tonometry and the EYEMATE-IO implant were performed.
Results
The EYEMATE-IO implant was successfully implanted in 22 patients with few surgical complications and no unexpected device-related AEs. All ocular AEs resolved quickly under appropriate treatment. Comparative measurements showed good agreement between EYEMATE-IO and Goldmann applanation tonometry (GAT) with an intraclass correlation coefficient (ICC(3,k)) of 0.783 (95% confidence interval [CI]: 0.743, 0.817). EYEMATE-IO measurements were higher than GAT, with a mean difference of 3.2 mm Hg (95% CI: 2.8, 3.5 mm Hg).
Conclusions
The EYEMATE-IO sensor was safely implanted in 22 patients and performed reliably until the end of follow-up. This device allows for continual and long-term measurements of IOP.
Intraocular pressure (IOP) is currently the only modifiable risk factor for the onset and progression of glaucoma. Therefore, accurate and reliable measurement of this parameter is the cornerstone of diagnostic and therapeutic decision making in glaucoma. Currently, best practice patterns in IOP evaluation are defined by infrequent measurements of approximately 3 to 6 times per year. It is known, however, that IOP is a highly dynamic biological parameter with short- and long-term fluctuations. The existence of individual, relatively stable circadian IOP profiles has been proposed by some studies but remains controversial. It is estimated that up to 80% of peak IOP occur outside of normal office hours, often during the nocturnal period. , , There is some evidence to suggest that IOP variability itself may be an independent risk factor for the development and progression of glaucoma. However, because of the unavailability of prospective studies using continuous 24-hour IOP monitoring technology, this question remains disputed with contrasting data.
Most methods for measuring IOP require trained personnel and specialized equipment, which limits IOP monitoring to ophthalmologists’ or optometrists’ offices. The current gold standard for measuring IOP is Goldmann applanation tonometry (GAT), which requires contact of a measuring probe to the cornea and thus the use of topical anesthetics. GAT has remained the standard despite its many shortcomings mainly because of its ease of use and low cost. One of the major sources of error in current tonometry is dependence on the biomechanical properties of the cornea, including central corneal thickness, corneal astigmatism, corneal curvature, and rigidity. There can be significant discrepancies between measured and true IOP, as well as between measurements in the same subject by different examiners. Some commercially available tonometry devices, such as the ocular response analyzer and dynamic contour tonometer, claim to compensate for corneal parameters. These are, however, either expensive or difficult to use, limiting their use in everyday clinical practice. Moreover, they do not solve the problem of “undersampling” IOP in the treatment of glaucoma.
The novel EYEMATE-IO sensor is an implantable device for continual IOP monitoring that is placed within the ciliary sulcus during cataract surgery. An external handheld reader enables on-demand measurements of IOP and wireless power supply of the device. The first-generation device has been shown to be safe and able to obtain IOP measurements in 7 eyes with up to 1 year of follow-up. The purpose of this open-label single-arm clinical investigation was to evaluate the safety and performance of the second-generation EYEMATE-IO sensor in patients with primary open angle glaucoma (POAG).
Methods
Study Design
The ARGOS-02 trial was designed as a prospective, open-label, single-arm, multicenter observational study, aimed at assessing the safety and performance of the EYEMATE-IO system in patients with POAG. The study adhered to the tenets of the Declaration of Helsinki, received prospective ethics committee/institutional review board approval at each study site, and was registered at clinicaltrials.gov ( NCT02434692 ).
The primary objectives were to evaluate the safety and tolerability of the EYEMATE-IO sensor by assessing the number and nature of serious adverse device-related events (SADEs) within the first 3 months after implantation. The primary performance endpoint was to evaluate the limits of agreement between measurements with the EYEMATE-IO and GAT up to 3 months after implantation.
Secondary safety endpoints included the incidence, nature, severity, and seriousness of all observed AES, including ADEs, in the 12 months following implantation. Secondary performance endpoints included the number and nature of device malfunctions, limits of agreement between EYEMATE-IO and GAT measurements, as well as assessment of diurnal IOP profiles obtained via self-guided self-measurements with EYEMATE-IO at home between study visits.
Safety and performance endpoints were assessed at regular prescheduled visits on days 1, 3, 10, 30, 60, 90, 120, 180, 240, and 360 after surgery. For safety assessment, a complete ophthalmologic exam including best corrected visual acuity, slit lamp biomicroscopy, funduscopy, external eye photography, and tonometry was performed at each visit and any AE or change in medication was recorded. Additionally, perimetry, gonioscopy, specular microscopy, anterior segment optical coherence tomography (OCT), posterior pole OCT, and fundus photography were performed at screening as well as on days 90, 180, and 360 after surgery. Starting on day 30 after surgery, either 2 or 4 series of comparative measurements between GAT and EYEMATE-IO were performed at each visit. GAT was measured before EYEMATE-IO to avoid influencing the subjective GAT readout. Additionally, any device deficiencies were recorded at any visit.
Description of Device
The EYEMATE-IO system comprises a ring-shaped foldable sensor device to be implanted into the ciliary sulcus during cataract surgery, an injector device for the facilitation of this implantation procedure, as well as an external handheld reader device (MESOGRAPH) for the activation, power supply, and readout of the implanted sensor.
Sensor ring
The EYEMATE-IO pressure sensor, which is intended for permanent implantation in the ciliary sulcus of the eye, bears a microelectromechanical system application-specific integrated circuit (ASIC) that integrates pressure and temperature sensors, identification, and analog-to-digital encoders and a telemetry unit. The ASIC is bonded to a gold microcoil and hermetically encapsulated in a ring of medical-grade silicone rubber material that has been validated through its previous use for intraocular lenses. An initial description of the underlying technology was published by Todani and associates. The final dimensions of the implant are an inner diameter of 7 mm and an outer diameter of 11.3, 11.7, or 12.1 mm, to be used depending on the size of the ciliary sulcus, a thickness of 0.9 mm at the ASIC, and a thickness of 0.5 mm around the microcoil (see Figure 1 ). These changes constitute a modification and miniaturization compared to the first-generation device. Further modifications include tapered edges on the outer ring as well as introduction of 4 small pedicles to act as haptics for secure but strainless placement in the ciliary sulcus.
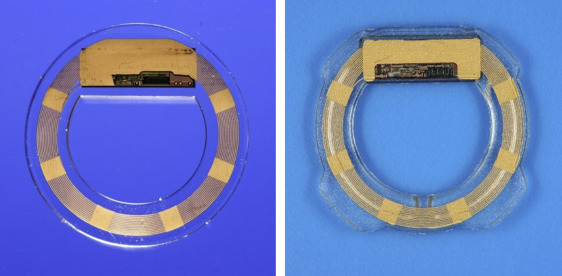
When the external reading device (Mesograph) is activated in close proximity to the eye (within approximately 5 cm), an electromagnetic inductive connection is formed between it and the microcoil that provides the ASIC with power and enables the measurement and telemetric data transfer between the sensor and the reader. Each sensor is calibrated individually to be highly accurate between absolute pressures ranging from 800 to 1150 hPa (normal atmospheric pressure) at physiological temperatures (30°C-42°C) before sterilization and packing. Each sensor is checked for plausible readings again immediately before and after implantation.
Injector device
The implantation procedure is performed using a multipart injector device that uses a single-use Teflon-based cartridge, holding the partly rolled-up sensor ring. The injector is used to facilitate placement of the sensor ring in the eye through the widened phacoemulsification tunnel to minimize surgical trauma. This is a departure from the original “manual” implantation method used for the previous study. The use of an injector was enabled by the reduction of thickness and rigidity of the sensor ring.
Mesograph—External reader device
The external reader device is a hand-held device that provides power to the sensor when activated within 5 cm of the eye. It comprises a power source (2CR5 lithium battery), a coil generating the electromagnetic field to power the sensor via electromagnetic coupling, which also acts as an antenna for the transmission of the signals provided by the sensor. The device also contains a separate pressure sensor measuring external atmospheric pressure (absolute) that is compared to the pressure reading provided by the sensor. The difference between both absolute pressures, expressed in mm Hg, represents the IOP, which is displayed on a LED display of the reader device. The reader can store up to 3000 individual IOP readings, which can be transferred to a computer via a cable connection or wirelessly through a GSM module.
Patient Population
Patients with cataract and concomitant POAG, who were regularly scheduled for cataract surgery, were asked to participate in the study based on the following inclusion and exclusion criteria.
Inclusion criteria
The inclusion criteria were as follows: mentally competent and willing to provide written informed consent; male or female aged ≥40 years and ≤85 years on the day of screening; diagnosis of POAG as defined by the European Glaucoma Society guidelines ; controlled IOP, as determined by treating clinicians; cataract surgery indicated irrespective of study participation; preoperative anterior chamber depth ≥2.0 mm as measured from the corneal endothelium; axis length >22 mm; corneal endothelial cell density ≥2000 cells/mm 2 .
Exclusion criteria
The exclusion criteria were as follows: any type of glaucoma other than POAG; severe visual field loss of –20 dB or worse in both eyes (In cases with different stages of disease, the sensor was to be implanted in the worse eye.); presence of other severe sight-threatening ocular conditions (eg, age-related macular degeneration, retinal detachment, ocular tumors, severe dry eye syndrome); corneal diseases, especially those affecting the corneal endothelium and conditions potentially affecting assessment of visual acuity and/or IOP by GAT; diabetes mellitus; history of connective tissue disease (ie, Marfan syndrome, Ehlers-Danlos syndrome, or Weill-Marchesani syndrome); history or evidence of severe inflammatory eye diseases (ie, uveitis, retinitis, scleritis) in one or both eyes within 6 months prior to EYEMATE-IO implantation; intraocular surgical procedure(s) within 6 months prior to EYEMATE-IO implantation or any surgical procedure with the potential to affect the assessment of IOP by GAT; anterior chamber configuration with high risk of an angle closure glaucoma; subjects with planned ancillary procedures in the study eye at the time of implantation or during the postoperative study period.
After informed consent was given for both cataract surgery and study participation, patients meeting all inclusion and exclusion criteria were scheduled for surgery at one of the 11 sites participating in the study.
Description of implantation procedure
The sensor ring is placed in the ciliary sulcus of the eye during routine cataract surgery. As the size of the implant and the sleeve of the injector tip used for this study required an incision width of approximately 6 mm, most sites chose a sclerocorneal tunnel (temporal approach) to the implantation. After clearing the conjunctiva, the tunnel was pre-formed without penetration of the anterior chamber. Depending on surgeon preference, 1 or 2 paracenteses were then performed using a standard 15° stab incision blade followed by filling of the anterior chamber with an ocular viscoelastic device (OVD) of high viscosity. The phaco tunnel was created using a standard phaco slit knife (2.8-3.2 mm), followed by regular capsulorrhexis, phaco-emulsification, and intracapsular placement of the intraocular lens. The posterior chamber was then widened by use of the same high-viscosity OVD, carefully avoiding too much OVD in the incision quadrant so as to prevent iris prolapse. The pre-formed sclerocorneal tunnel was then widened to fit the sleeve of the injector device, which was either inserted into or docked onto the tunnel opening. The sensor ring was then inserted into the posterior chamber and guided into the sulcus using a flat atraumatic spatula through a paracentesis, carefully avoiding touch of the sensor’s ASIC. The OVD was then removed, the pupil constricted medically, the sclerocorneal tunnel sutured, the globe toned to intermediate IOP and the conjunctiva reattached with sutures. Figure 2 shows the injection and unfolding of the sensor ring.

Results
Patient characteristics
A total of 24 patients with POAG and concurrent cataract were initially enrolled into this clinical trial, 22 of whom successfully received the EYEMATE-IO. Of the 2 patients initially enrolled but not implanted, 1 was excluded because of a screening failure. In the second patient, after surgical complications early during cataract surgery, an implantation of the EYEMATE-IO sensor was not attempted. Thus, exclusion of this patient was unrelated to the EYEMATE-IO device. A total of 22 sensors were used for this study, with no device deficiencies occurring during the implantation procedure. In all implanted patients, the sensors remained in the eye to the end of the study.
At enrollment, patients (8 female and 14 male) were between 55 and 78 years of age (mean 67.8 years, SD 6.8 years) and had been diagnosed with glaucoma for up to 29 years (mean duration 8.74 years, SD 7.94 years). At the time of enrollment, 17 subjects were using at least 1 anti-glaucoma medication. Ten patients had concurrent ocular conditions other than glaucoma and cataract and had undergone other surgical ophthalmic procedures, including laser procedures, prior to enrollment. Two of the patients had had previous glaucoma surgery in the study eye: trabeculectomy and selective laser trabeculoplasty in one patient and canaloplasty in the other patient.
Safety
Surgical complications
Surgical complications were reported in 7 of 23 patients. In 5 of these patients, complications occurred during the implantation of the EYEMATE-IO device. The complications that occurred most often (5 times each) were iris prolapse/floppy iris and pigment dispersion. Both flat anterior chamber and “vis a tergo” (“pressure from behind”) were reported twice. All surgical complications occurred early in the study. After re-evaluation of the surgical procedure and retraining of the surgeons, no further complications occurred during surgery.
Adverse events
Four serious ocular AEs were reported for the study after implantation. These included 2 cases of fibrin reaction in the anterior chamber, 1 case of temporary corneal decompensation, and 1 case of intractable IOP increase requiring subsequent glaucoma surgery. This patient underwent trabeculectomy 345 days after initial surgery. Although it cannot be ruled out that pigment dispersion due to iris manipulation during the implantation procedure, which was reported as an adverse event in this case, contributed to increased IOP in this patient, the cause of the increase could not be conclusively determined.
The 2 cases of severe fibrin reaction (7 and 13 days after initial implantation) lead to precautionary hospitalization because of the novelty of the device and procedure, but resolved quickly and without sequelae under intensive anti-inflammatory treatment. No incidences of hypopyon or uveitis were reported.
One patient was treated for corneal decompensation 9 days after surgery (treatment period 22 days). Careful review of the implantation process revealed a complicated surgical procedure with potential corneal touch of either the IOL or the sensor implant. In all, 70 nonserious AEs in 18 patients were considered to be at least possibly related to the medical device, and 90 AEs in 21 patients had a potential relationship to the medical procedure at initial assessment. These included increased IOP (22 times in 14 patients), anterior chamber inflammation with increased anterior chamber cells and Tyndall flare (11 times in 9 patients), and mild to moderate pigment dispersion in 8 patients. One case of persistent cystoid macular edema was treated with nonsteroidal anti-inflammatory drugs for 7 months and resolved without sequelae.
Visual acuity 3 months after surgery was better than at screening owing to removal of the cataract. Best corrected visual acuity increased from 65.0 ± 21.6 ETDRS letters before surgery to 77.5 ± 13.2 letters 3 months after surgery ( P = .0045) and remained stable throughout the trial: at follow-up visit 11 (day 360), it was recorded at 79.8 ± 13.5 ETDRS letters ( P = .0012).
The mean endothelial cell density decreased from 2403 ± 198 cells/mm 2 at screening to 2177 ± 438 cells/mm 2 at the end of follow-up, constituting a loss of 9.4% over the course of 12 months ( P = .0502). This higher than expected cell loss mainly stems from one patient who suffered temporary corneal decompensation after corneal touch during surgery, whose endothelial cell density dropped from 2361 to 1599 cells/mm 2 before stabilizing. Central corneal thickness remained stable throughout the study. There was mild to moderate corneal swelling and turbidity in the early postoperative phase in half of the patients (11 of 22), with mild to moderate Descemet folds resolving in most cases before follow-up visit 5 (day 30).
There was a slight decrease in anterior chamber angle from median Shaffer grade 3 at screening to a median Schaffer grade 2 at the end of study with an increase in median pigmentation from mild to moderate over the course of the study. No case of angle closure or anterior synechiae occurred. Mild to moderate iris transillumination defects were seen in 8 patients and mild pigment deposits in the anterior chamber in 6 patients at the end of the study. These were most likely attributable to iris manipulation during the implantation procedure, as they were seen either already during, or immediately after, surgery and stabilized after the initial postoperative healing phase. A typical example is shown in Figure 3 , placement of the sensor is shown in Figure 4 .





