OVERVIEW OF TERMS AND MECHANISMS
The diseases discussed in this chapter share a final common pathway of angle closure and obstruction of outflow, but they are associated with contributory or causative conditions beyond the parameters elaborated in the previous chapter for the spectrum of primary angle-closure glaucoma (PACG). They are thus distinct from PACG, the most common cause of glaucoma blindness in the world. When a related or identifiable ophthalmic condition is known to be present with the onset of angle closure, it is referred to as secondary . We propose that this definition now subsumes and replaces the category of ‘combined (mixed) mechanism glaucoma’ as well. It is important for clinicians to be familiar with these secondary angle-closure conditions, not because they are common causes of glaucoma, but rather because they are capable of producing severe elevations of intraocular pressure (IOP) and marked loss of vision.
Historically, ‘combined (or mixed) mechanism glaucomas’ referred to eyes with more than one diagnosis, identified either coincidentally or sequentially. Their angles are not usually seen as closed, yet they present with compromised outflow. In some cases the existence of two types of glaucoma in one eye appears to be a chance occurrence; for example, an eye with exfoliation syndrome suffers trauma and develops uveitis with secondary angle-closure glaucoma. In other cases the treatment of one form of glaucoma produces a second form of the disease; for example, a filtering procedure for juvenile open-angle glaucoma is complicated by a flat anterior chamber, extensive peripheral anterior synechiae (PAS), and progressive glaucoma which is now due to angle closure. It seems unnecessary to perpetuate the anecdotal and highly idiosyncratic nomenclature of the ‘combined mechanism’ categories. When discrete causative factors are known – such as PAS following laser trabeculoplasty for primary open-angle glaucoma (POAG) – such cases can be conceptualized as ‘secondary angle-closure glaucomas’, and etiologic mechanisms identified according to the explanatory model elaborated below.
It is easiest to conceptualize the secondary angle-closure glaucomas as occurring through two different fundamental mechanisms: an anterior pulling mechanism and a posterior pushing mechanism . With the anterior pulling mechanism, the peripheral iris is pulled forward onto the trabecular meshwork by the contraction of a membrane, inflammatory exudate, or fibrous band ( Fig. 16–1 ). Examples of this mechanism include neovascular glaucoma and the iridocorneal endothelial (ICE) syndrome. As the membrane, band, or inflammatory material contracts, it acts like a zipper to form permanent PAS, which can be spotty and irregular or diffuse and quite regular. Pupillary block plays little or no role in this mechanism.

With the posterior pushing mechanism, the peripheral iris is displaced forward by the lens, vitreous, or ciliary body ( Fig. 16–2 ). An example of this mechanism occurs when gas is injected into the vitreous cavity to repair a retinal detachment, displacing the lens–iris diaphragm sufficiently forward to close the angle. This can happen despite the presence of a patent iridotomy.

The degree of pupillary block in the secondary angle-closure glaucomas is, by definition, not the primary and exclusive event. The posterior pushing mechanism in particular can be accompanied by varying degrees of pupillary block. In some conditions, such as ciliary block glaucoma (aqueous misdirection or malignant glaucoma) or in retinopathy of prematurity, pupillary block needs to be eliminated with iridotomy for both diagnostic and therapeutic reasons ( Fig. 16–3 ).

ANTERIOR PULLING MECHANISM
NEOVASCULAR GLAUCOMA
Neovascular glaucoma is caused by a fibrovascular membrane that develops on the surface of the iris and the angle. At first the membrane merely covers the angle structures, but then it contracts to form PAS. Neovascular glaucoma is virtually always associated with other ophthalmic abnormalities, most commonly some form of ocular ischemia. Neovascular glaucoma is an important entity because it often causes great morbidity and visual loss. A variety of other terms have been used to describe this condition, including thrombotic glaucoma, hemorrhagic glaucoma, diabetic hemorrhagic glaucoma, congestive glaucoma, and rubeotic glaucoma. The term neovascular glaucoma is used here because it includes all glaucoma caused by or related to a fibrovascular membrane on the iris and/or angle. It is important to distinguish the terms neovascular glaucoma and rubeosis iridis . Rubeosis iridis refers to new vessels on the surface of the iris regardless of the state of the angle or the presence of glaucoma.
Neovascular glaucoma was first described in 1866 following central retinal vein occlusion. Additional descriptions were provided by various observers in the latter part of the nineteenth century and early twentieth century, including Coates in 1906. Nettleship and Salus noted the association of neovascular glaucoma and diabetes mellitus. Kurz described the gonioscopic appearance of new vessels in the angle and postulated that this fibrovascular tissue contracted to form PAS. Until 1963, the condition was known mostly as ‘hemorrhagic glaucoma’, based on the occasional association with hyphema; the term ‘neovascular glaucoma’ was proposed by Weiss and co-workers, and because this term better fits with the pathophysiology of the condition, it has become the accepted one.
Histopathology
Histopathologic examination of eyes with neovascular glaucoma, regardless of etiology, reveals that the new vessels arise from the microvascular bed (capillaries or venules) in the iris and ciliary body. The new vessels appear first as endothelial buds from capillaries of the minor arterial circle; new buds may then appear from vessels anywhere in the iris. Changes occur within the microstructure of the endothelial cells and in the extracellular matrix surrounding them. The buds then become vascular tufts not unlike tiny glomeruli. The new vessels have thin walls with irregular endothelia and pericytes. The junctions between the endothelial cells appear to be open, which accounts for their leakiness on angiography.
With time, a clinically invisible fibrous membrane develops along the vessels. The membrane contains myofibroblasts that have contractile properties. The contraction of the myofibroblasts pulls the posterior pigment layer of the iris epithelium anteriorly, producing ectropion uveae, and pulls the peripheral iris into the chamber angle, producing PAS. There is one report that new vessels developing after central retinal vein occlusion are larger in diameter and more irregular than those associated with diabetes mellitus.
Despite the variety of underlying diseases, the clinical appearance and histopathologic findings of neovascular glaucoma do not vary greatly. However, there may be some variation in the acuteness of the onset and the rate of progression, depending on the underlying condition. For example, neovascular glaucoma associated with central retinal vein occlusion may progress more rapidly than that associated with diabetes mellitus, and often depends on the predominance of either an ischemic or hemorrhagic retinal insult.
Pathogenesis
The pathogenesis of neovascular glaucoma is that retinal ischemia liberates angiogenic factors that diffuse forward and induce new vessel formation on the iris and in the angle. Capillary occlusion or ischemia appears to be the initiating event in this process, which seems to be similar to the production of an angiogenic factor or factors by solid tumors. Angiogenic substances (or substance) from tumors implanted in the eye but at a distance from the retina and iris have been shown to cause neovascularization of both. Angiogenic factors are produced by hypoxic retinal tissue in vitro . Angiogenic factors have been detected in mammalian retina and in aqueous humor samples from patients with neovascular glaucoma. In laboratory experiments, this factor (or factors) is capable of stimulating capillary endothelial proliferation, corneal neovascularization, and retinal neovascularization. Vasoproliferative factors have been detected in increased amounts in the eyes of both animal models and patients with neovascular glaucoma. And most recently is the impressive regression of new vessels induced by molecular-specific antiangiogenic factors, such as bevacizumab.
Historically, a number of substances have been proposed as the angiogenic factor. Some of the families of compounds having angiogenic activity include fibroblast growth factor, vascular endothelial growth factor (VEGF), angiogenin, platelet-derived endothelial cell growth factor, transforming growth factor-α, transforming growth factor-β, and tumor necrosis factor-α. Vascular endothelial growth factor is thought to be a primary culprit. It is found in concentrations 40–100 times normal in the aqueous humor of patients with neovascular glaucoma. Tolentino and co-workers showed that intravitreal injection of VEGF can produce iris neovascularization and neovascular glaucoma in primates. Some consider that angiogenesis is most likely a process (not unlike the clotting or inflammatory cascades) involving several families of agents, including polypeptides, amines, lipids, and other low molecular weight compounds.
The above theory explains many observations about neovascular glaucoma. Diffusible molecules from the retina enter the anterior chamber through the pupil, with their highest concentration near this site. This may explain the initial appearance of rubeosis iridis at the pupillary margin. This mechanism also accounts for why rubeosis iridis and neovascular glaucoma are more common after cataract extraction with capsular disruption and after vitrectomy in eyes with vascular retinopathy. The lens and vitreous may serve as mechanical barriers to the diffusion of an angiogenic substance. Furthermore, the vitreous humor apparently also contains an endogenous inhibitor of angiogenesis. Finally, vitrectomy and cataract surgery cause inflammation, which may further serve as a stimulus to neovascularization. Lastly, this theory explains the efficacy of panretinal photocoagulation or retinal cryoablation in neovascular glaucoma, treatments which destroy ischemic retina that had been synthesizing the angiogenic factor(s). Aiello and co-workers have found a marked decrease in VEGF in the vitreous of patients after panretinal photocoagulation. These treatments may also liberate inhibitory factors that counteract the vasoproliferative stimulus.
Conditions and diseases commonly associated with neovascular glaucoma
Neovascular glaucoma is associated with a large number of diseases and conditions ( Box 16–1 ). As noted above, most of these conditions have some relation to either retinal or ocular ischemia or to chronic inflammation. In a large comprehensive survey, diabetes mellitus was associated with about one-third of the cases of neovascular glaucoma; central retinal vein occlusion with another third; and a variety of conditions with the last third – with carotid occlusive disease being the most common in the last group. The discussion here is limited to a few of the more common entities, such as central retinal vein occlusion and diabetes mellitus, from among a wide variety of predisposing conditions.
Ocular vascular disease
Central retinal vein occlusion
Central retinal artery occlusion
Branch retinal vein occlusion
Branch retinal artery occlusion
Sturge-Weber syndrome with choroidal hemangioma
Leber’s miliary aneurysms
Sickle cell retinopathy
Diabetes mellitus
Extraocular disease
Carotid artery disease/ligation
Ocular ischemia
Aortic arch syndrome
Carotid-cavernous fistula
Giant cell arteritis
Pulseless disease
Assorted ocular diseases
Retinal detachment
Eales’ disease
Coats’ disease
Retinopathy of prematurity
Persistence and hyperplasia of the primary vitreous
Retinoschisis
Glaucoma
Open-angle
Angle-closure
Secondary
Norrie’s disease
Stickler’s syndrome
Trauma
Essential iris atrophy
Neurofibromatosis
Lupus erythematosus
Marfan’s syndrome
Recurrent hemorrhages
Vitreous wick syndrome
Ocular neoplasms
Malignant melanoma
Retinoblastoma
Optic nerve glioma associated with venous stasis
Metastatic carcinoma
Reticulum cell sarcoma
Medulloepithelioma
Squamous cell carcinoma conjunctiva
Angiomatosis retinae
Ocular inflammatory disease
Chronic uveitis
Endophthalmitis
Sympathetic ophthalmia
Syphilitic retinitis
Vogt-Koyanagi-Harada syndrome
Ocular therapy
Cataract excision (especially in diabetics)
Vitrectomy (especially in diabetics)
Retinal detachment surgery
Radiation
Laser coreoplasty
Modified from Gartner S, Henkind P: Neovascularization of the iris (rubeosis iridis), Surv Ophthalmol 22:291, 1978 and Wand M: Neovascular glaucoma. In: Ritch R, Shields MB, Krupin T, editors: The glaucomas, 2nd edn., St Louis, Mosby, 1982.
Diabetes mellitus
Diabetes mellitus is one of the most common causes of neovascular glaucoma, accounting for approximately one-third of the cases. Neovascular glaucoma is usually seen in eyes with proliferative diabetic retinopathy, but it can be seen in eyes with nonproliferative retinopathy if there are large areas of capillary nonperfusion. The prevalence of neovascular glaucoma is related to the duration of diabetes and may also be influenced by the presence of other vascular diseases such as hypertension. It is common for neovascular glaucoma to appear within 6 months of vitrectomy in diabetic patients, especially in aphakic eyes, in eyes with proliferative retinopathy, and in eyes with pre-existing rubeosis iridis. In similar fashion, diabetic neovascular glaucoma is a common occurrence after intracapsular cataract extraction, whether performed alone or in combination with vitrectomy. There is evidence that diabetic neovascular glaucoma is less common after extracapsular cataract extraction than after intracapsular cataract extraction, unless the capsule is ruptured, or zonular support is lost with exposure of vitreous (as seen with lax capsular support in pseudoexfoliation.) As noted previously, the lens and vitreous may act as mechanical barriers to the forward movement of angiogenic factors elaborated by the retina. The vitreous may also serve as an endogenous inhibitor of angiogenic stimuli.
It is especially important to emphasize the distinction between rubeosis iridis and neovascular glaucoma in diabetic eyes. Rubeosis iridis is said to occur in 1–17% of diabetic eyes, and in 33–64% of eyes with proliferative diabetic retinopathy. Clearly, the prevalence of rubeosis iridis is much higher than the prevalence of neovascular glaucoma. Rubeosis iridis may progress to neovascular glaucoma in some diabetic patients, but in others the condition remains stationary for long periods of time or even regresses. The rate of progression is much lower if the retina is treated with photocoagulation. If neovascular glaucoma develops in one eye of a diabetic patient, the fellow eye is at high risk if adequate retinal photocoagulation is not applied.
Central retinal vein occlusion
Central retinal vein occlusion is among the commonest cause of neovascular glaucoma. It is estimated that about 30% of patients who suffer a central retinal vein occlusion develop neovascular glaucoma. More comprehensive investigations have done much to clarify this association. Hayreh has carefully discriminated central retinal vein occlusion into two types – ischemic and non-ischemic (venous stasis retinopathy). Approximately three-quarters of central retinal vein occlusions are non-ischemic, and one-quarter are ischemic. Yet neovascular glaucoma occurs in 18–86% of eyes with ischemic vein occlusions, as opposed to 0–4% of eyes with non-ischemic occlusions. According to a large study, about 40% of patients with ischemic central retinal vein occlusion will develop neovascular glaucoma. The distinction between ischemic and non-ischemic vein occlusions is usually made by judging the degree of retinal capillary non-perfusion (capillary dropout) on fluorescein angiography. Other signs of ischemia include 10 or more cotton wool spots in the retina, an absent perifoveal capillary network on fluorescein angiography, arteriovenous transit time greater than 20 seconds, leaky iris vessels on angiography, and a reduced B:A wave ratio on electroretinography. Eyes with ischemic central retinal vein occlusions should receive panretinal photocoagulation (or cryoablation if no laser is available) to reduce the incidence of neovascular glaucoma. Careful follow-up is mandated even in those with the non-ischemic type because of the observation that one-third of eyes with central retinal vein occlusion and good perfusion at the onset show signs of ischemia by 3 years.
Neovascular glaucoma may present anywhere from 2 weeks to 2 years following a central retinal vein occlusion. However, the condition often presents about 3 months after central retinal vein occlusion: hence its reputation as the ‘100-day glaucoma’. Younger patients with central retinal vein occlusions often have associated vascular diseases, such as hypertension or one of the collagen vascular disorders.
Older patients with central retinal vein occlusions often have associated glaucoma or elevated IOP. Elevated IOP or glaucoma has been reported in 10–23% of eyes that developed a central retinal vein occlusion. In most cases the underlying glaucoma is open angle or exfoliative in type, but there have been a few reports of central retinal vein occlusion following angle-closure glaucoma. The underlying glaucoma is often masked because these eyes may have a low IOP for weeks to months following vein occlusion. In addition, the low IOP may reflect transient poor perfusion of the ciliary body. The presence of pre-existing POAG increases the risk of neovascular glaucoma after central retinal vein occlusion despite adequate prophylactic laser treatment: adequate treatment of the pre-existing glaucoma does not prevent the onset of neovascularization. Furthermore, pre-existing open-angle glaucoma may make any subsequent neovascular glaucoma more refractory to treatment. It is also common for fellow eyes to have elevated IOP or to develop it at a later time. Although a true causative role for elevated IOP in central retinal vein occlusion has not been established, it is probably wise to treat fellow eyes with elevated IOP with ocular hypotensive agents. One case-control study does support elevated IOP as a risk factor in central retinal vein occlusion along with systemic hypertension and male gender. Green and co-workers proposed that posterior bowing of the lamina cribrosa in glaucoma creates a mechanical obstruction that impedes the venous outflow and contributes to venous stasis and/or occlusion.
Carotid occlusive disease
Carotid artery disease is considered the third most common cause of neovascular glaucoma. Neovascular glaucoma has been reported after carotid artery ligation and idiopathic carotid artery obstruction. The obstruction can be unilateral or bilateral and can involve the common carotid artery or the internal carotid artery. Carotid artery obstruction does not cause neovascular glaucoma in all cases because there is usually sufficient collateral flow to prevent widespread retinal ischemia. Carotid artery palpation and auscultation should be performed in all cases of central retinal vein occlusion. Neovascular glaucoma associated with carotid artery disease often has a confusing presentation and a variable course. If anterior segment ischemia is severe, the vessels on the iris may be less visible, and the IOP may be normal or even low despite extensive neovascular closure of the angle. These eyes often suffer wide swings in IOP, depending on the perfusion to the ciliary body. Patients who undergo surgery to relieve or bypass carotid artery obstruction may experience a dramatic rise in IOP when the ciliary body blood supply improves and aqueous humor formation increases. Panretinal photocoagulation may be less successful in eliminating iris neovascularization in patients with carotid artery obstruction because the anterior segment of these eyes is also ischemic and is not affected by retinal ablation techniques.
Ocular ischemic syndrome
Several authors have described a condition called chronic ocular ischemic syndrome that includes signs of transient ischemic attacks; ocular motor disturbances; midperipheral retinal hemorrhages; neovascularization of the iris; and, late in its course, corneal striae and hypotony. Although the term was initially used to describe obstruction of the carotid artery, extracarotid causes have also been identified, including abnormalities of carotid flow without stenosis, cranial arteritis, and coronary artery disease. Carotid artery obstruction accounts for about 75% of these patients, with other risk factors being diabetes mellitus, systemic hypertension, and a history of cerebrovascular accident. Doppler imaging of the carotids should be considered if any of the symptoms or signs associated with the ocular ischemic syndrome are manifest. Treatment proposals have included oral verapamil, panretinal photocoagulation, and carotid endarterectomy, although the latter intervention does not always effect a significant clinical improvement.
Central retinal artery occlusion
Other vascular occlusive diseases of the eye may also be associated with neovascular glaucoma. Seven per cent to 15% of patients with a central retinal artery occlusion will develop neovascular glaucoma. Some, but not all, have concomitant carotid artery occlusive disease. Again, the preponderance of ischemic and disrupted retina is felt to be causative. Although panretinal photocoagulation may have some effect in reducing the incidence of neovascular glaucoma, it is not as effective as in retinal vein occlusion or diabetes mellitus.
Miscellaneous
Neovascular glaucoma occurs after a variety of therapeutic interventions, including radiotherapy, microwave thermoradiotherapy, and retinal detachment surgery. Neovascular glaucoma occurs after radiation therapy for uveal melanoma between 8.5% and 25% of the time and is dose and time dependent. Cataract extraction in eyes that have had radiation is a risk factor for the acceleration of neovascular glaucoma, as it is in eyes with diabetes. Clearly, many of the eyes requiring these therapies have underlying diseases producing ischemia of the retina or tumors capable of producing vasoproliferative factors. Intraocular tumors also can be associated with neovascular glaucoma.
Clinical presentation
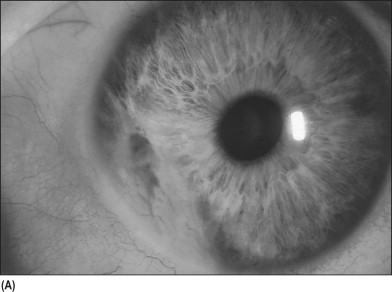
Neovascular glaucoma often presents with an acute onset of pain, tearing, redness, and blurred vision. In some cases, depending on the underlying disease, patients report diminished vision for weeks to months before the onset of the pain and redness. When first seen, an affected eye may have ciliary injection, a hazy cornea from epithelial edema, a deep anterior chamber with moderate flare, a hyphema, a small pupil, and new vessels on the iris and in the angle. The first sign of rubeosis iridis is increased permeability of the blood vessels at the pupillary margin as detected by fluorescein angiography or fluorophotometry. Clinically the new vessels are first detected as small tufts at the pupillary margin. Occasionally new vessels are seen first in the angle if the tufts near the pupil are obscured by dark iris pigment ( Fig. 16–4A ). The neovascularization progresses over the iris surface and into the angle. The new vessels extend from the iris root across the ciliary body and scleral spur, where they arborize over the trabecular meshwork ( Fig. 16–4B ). At times it may be difficult to distinguish new vessels from normal iris vessels, especially in inflamed eyes. Normal iris vessels have a uniform size and a radial course, and they do not branch within the iris. In contrast, new vessels have an irregular size and an irregular course, and they branch frequently. New vessels also lie on the iris surface rather than in the stroma as normal vessels do.

When the fibrovascular membrane covers a substantial portion of the trabecular meshwork, outflow facility falls and IOP rises. With time, the membrane pulls the peripheral iris up into the angle ( Fig. 16–4C ). The rate at which this occurs is quite variable, ranging from days to years.
In the late stages of neovascular glaucoma the eye is painful with bullous keratopathy, a sealed angle, and intractable glaucoma. Traction from the fibrovascular membrane lifts the iris anteriorly, gives the stroma a compacted appearance, and produces ectropion uveae and a fixed, dilated pupil. At this stage, the new vessels may be much less visible, especially those in the angle ( Fig. 16–4D ).
Treatment
Historically, eyes with neovascular glaucoma had a poor prognosis, and enucleation for chronic pain was a frequent outcome. This dismal picture has changed remarkably over the past several decades, and in many instances neovascular glaucoma can be prevented or treated satisfactorily in terms of patient comfort, although restoration of visual function is uncommon.
Despite recent improvements in therapy, it is always far more effective to prevent neovascular glaucoma than to treat the disease once it is established. We have already mentioned the use of prophylactic panretinal photocoagulation (or retinal cryoablation) to deter neovascular glaucoma following ischemic central retinal vein occlusion. In similar fashion, photocoagulation can prevent neovascular glaucoma in eyes with proliferative diabetic retinopathy or non-proliferative retinopathy and large areas of capillary non-perfusion. Immediate panretinal photocoagulation may also prevent rubeosis iridis from progressing to neovascular glaucoma.
When a patient is seen with an acute episode of neovascular glaucoma, including markedly elevated IOP, the initial treatment consists of maximal IOP-reduction medical therapy, atropine for relief and to maximally dilate the pupil before iris mobility is lost, and corticosteroid. In this situation, miotic agents, prostaglandins, and adrenaline (epinephrine) are usually ineffective in lowering IOP, and may exacerbate pain and conjunctival injection. In most cases it is important to proceed rapidly with panretinal photocoagulation or retinal cryoablation to prevent total angle closure. Following this treatment, new vessels in the angle begin to regress within a few days to a few weeks. Depending on the extent of the PAS, the retinal treatment may abort the glaucoma, or leave a stable form of residual angle closure that may be responsive to medical therapy or surgery.
If extensive PAS are present after retinal ablation, and if IOP is not controlled by medical treatment, the clinician’s choice of therapy is usually based on the visual potential of the eye. If the eye has good visual potential, and if the neovascular membrane has regressed, filtering surgery can be successful, especially when augmented with antimetabolites. Wet field cautery or underwater diathermy to the sclera and iris may be useful to reduce intraoperative bleeding; but particularly helpful is preoperative intravitreal bevacizimab. Postoperatively, these eyes are often inflamed and require extensive topical, periocular, and systemic corticosteroid treatment.
Other authorities believe that there is a high failure rate of standard filtering surgery in eyes with neovascular glaucoma, even after the angiogenic stimulus has been reduced or eliminated, and despite antimetabolite agents. Today, many clinicians attempt to control the glaucoma with some type of posterior glaucoma drainage device. Glaucoma drainage implants appear to be successful in controlling pressures and preserving vision in approximately two-thirds of patients with neovascular glaucoma, dramatically reducing the need for enucleation in these otherwise doomed eyes. In aphakic eyes it is possible to implant the tube through the pars plana, so long as the anterior vitreal skirt has been removed by pars palna vitrectomy.
There are a number of therapeutic options for eyes with neovascular glaucoma and poor visual potential. Eyes with limited or no vision can often be made comfortable using cycloplegic agents and topical corticosteroids regardless of the IOP. Cyclodestructive procedures may be appropriate if the patient is too infirm for surgery, has too little visual potential to procede with filtration or tube surgery, or requires immediate pain relief. The history of attempting to reduce aqueous production by means of ciliary destruction is a long one.
Cyclocryotherapy often reduces IOP and makes patients more comfortable after an initial period of pain lasting 1–7 days, but this treatment is less effective in maintaining vision. Cyclocryotherapy is usually applied at −60°C to −80°C, using a large-tip probe with its anterior edge 2.5 mm posterior to the limbus. Six to eight 60–second freezes are placed over half of the circumference of the ciliary body. Frequent complications of this treatment include iridocyclitis, hypotony, pain, cataract, and phthisis bulbi. If cyclocryotherapy fails to reduce IOP, the treatment can be repeated over the same quadrants of the ciliary body and extended slightly. At least one-quarter of the ciliary body should remain untouched to reduce the incidence of phthisis bulbi. In the past, cyclodiathermy was used for the same purpose as cyclocryotherapy. Cyclodiathermy was largely abandoned and replaced by cryotherapy, which had a higher rate of success and a lower rate of complications. More recently, cyclocryotherapy itself has been replaced by either trans-scleral laser cyclophotocoagulation or endocyclophotocoagulation. One can expect approximately 65% success after 1 year in controlling pressures and pain with this modality. The results seem comparable with those achieved with posterior glaucoma drainage device implantation. In our hands, diode laser cyclodestruction with a contact delivery probe is a relatively safe and effective procedure for patients with poor vision or poor visual prognosis and for those for whom a glaucoma drainage device operation may be inadvisable (e.g., previous encircling band, poor physical condition). Retrobulbar alcohol injections and enucleation are appropriate treatments for eyes either with no useful vision or with intractable pain that does not respond to medical therapy and ciliodestructive procedures.
Some have advocated direct laser treatment to new vessels in the angle, a technique referred to as goniophotocoagulation, if neovascularization of the iris is encountered before PAS have formed. Low-energy argon laser treatments (0.2 seconds, 50–100 μm, 100–200 mW) are applied to the neovascular tufts as they cross the scleral spur. The laser therapy often must be repeated because these vessels may re-open minutes to days after treatment. Although goniophotocoagulation is inadequate treatment for neovascular glaucoma by itself, it may be a useful adjunct to panretinal photocoagulation in certain situations. For example, goniophotocoagulation can reduce angle neovascularization and synechia formation temporarily while panretinal photocoagulation takes effect and reduces the angiogenic stimulus. Finally, goniophotocoagulation can be applied when full panretinal photocoagulation is not totally successful in reducing the angiogenic stimulus. Its efficacy, however, is perhaps less than that of intravitreal angiogenic inhibitors; both modalities, however, may provide only temporary relief of weeks to months.
In neovascular glaucoma eyes secondary to central retinal vein occlusion, clinicians recommend intravitreal bevacizumab (1.25 mg/0.05 ml) (Avastin) to be administered through the pars plana 24–78 hours preceding surgery, with near total regression of iris neovascularization within 48 hours and some IOP lowering, an effect lasting for some weeks. This rapid regression of new vessels allows both for panretinal photocoagulation and glaucoma surgery with reduced risk of bleeding. Similarly focal laser or intraocular surgery can be enhanced with temporary regression of new vessels by such intervention. When used with combined surgical approaches such as pars plana vitrectomy with panretinal endolaser and filtration surgery, the outlook for greater surgical success in treating neovascular glaucoma in the short-to-medium term appears brighter.
IRIDOCORNEAL ENDOTHELIAL SYNDROME
The iridocorneal endothelial (ICE) syndrome takes many clinical forms but usually includes some combination of iris atrophy, corneal edema, and secondary angle-closure glaucoma without pupillary block. This syndrome is caused by an abnormal corneal endothelium that forms a membrane over the anterior surface of the iris and the angle structures. When this membrane contracts, it distorts the iris and closes the angle ( Fig. 16–5 ).




Histopathology
Histopathologic examination of eyes affected by the ICE syndrome reveals a thin, abnormal corneal endothelium and Descemet’s membrane separated by a thick accumulation of collagen. These tissues form a multilayered membrane that covers the angle and extends onto the anterior surface of the iris. The endothelial cells develop the epithelial-like characteristics of desmosomes, microvilli, tonofilaments (contractile elements), and proliferation – none of which occur in normal corneal endothelium. Most authorities believe this to be a metaplasia of endothelium into cells with epithelial characteristics, from an unknown trigger. These histopathologic changes are manifest on clinical examination by a ‘beaten silver’ appearance to the endothelium on slit-lamp examination; a loss of the normal, regular endothelial mosaic on specular reflection; and alterations in the size and shape of endothelial cells on specular microscopy. The size and shape of endothelial cells show great variation – some may be necrotic, and the findings are often patchy in the early stages of the condition. Even with the variation in clinical presentation, the endothelial findings are usually there if looked for carefully enough.
Pathogenesis
The most commonly accepted theory on the pathogenesis of the ICE syndrome proposes that the fundamental defect is in the corneal endothelium, whose dysfunction results in corneal edema. Furthermore, the corneal endothelium in this condition elaborates a membrane which causes a secondary angle closure. When the membrane contracts, it forms PAS leading to glaucoma, as well as iris defects such as corectopia, ‘stretch holes’, and iris nodules. Ischemia may be a secondary phenomenon producing ‘melt holes.’ At present we do not understand what causes the corneal endothelium to behave in this unusual manner. A few investigators postulate that there is an abnormal proliferation of neural crest cells or a fetal crest of epithelial cells. Other authorities suggest the endothelium proliferates because of inflammation. Electron micrographic, immunohistochemical, and serologic studies have suggested herpes simplex virus and, in another laboratory, Epstein-Barr virus. The viral theory is attractive and might explain the unilaterality of this syndrome in the vast majority of patients. In the past it was proposed that the ICE syndrome was a primary iris defect or that the disease occurred because of vascular insufficiency. These latter theories no longer seem tenable.
Clinical presentation
The PAS are more extensive in the quadrant toward which the pupil is displaced. The iris in the opposite quadrant has thinner stroma and full-thickness holes in some cases. In the Cogan-Reese syndrome, pigmented lesions project anteriorly from the iris surface and are surrounded by the multilayered membrane. The nodules are actually small portions of iris stroma that have been pinched off by the membrane.
Within the spectrum of the ICE syndrome there are three well-characterized clinical entities – progressive iris atrophy, Chandler’s syndrome, and Cogan-Reese syndrome – as well as a variety of intermediate forms. All of the variants of this syndrome appear in early to mid adult life, occur in whites more often than blacks, and affect women more commonly than men. The patients usually are seen after noticing a change in the appearance of their iris or pupil, a disturbance in their vision, or mild ocular discomfort. Although there are a few reports of familial cases, in most individuals the medical and family histories are unrevealing. The ICE syndrome almost always involves one eye, although the fellow eye may have subclinical abnormalities of the iris or corneal endothelium. Furthermore, there have been a few well-documented reports of individuals with bilateral involvement. Some degree of corneal endothelial abnormality is usually seen on slit-lamp examination in most patients with abnormal specular microscopy in all.
Progressive (essential) iris atrophy
In progressive iris atrophy (known as essential iris atrophy in the past) the clinical picture is dominated by corectopia and progressive dissolution of the iris ( Fig. 16–6 ). The iris dissolution begins as a patchy disappearance of the stroma and progresses to full-thickness holes ( Fig. 16–7 ). Some of the holes occur in quadrants away from the direction of pupillary displacement and are thought to be caused by traction (‘stretch holes’). Other holes occur without corectopia and are ischemic in nature (‘melt holes’), as demonstrated on fluorescein angiography of the iris. Broad patchy PAS form attachments anterior to Schwalbe’s line. The synechiae lift the iris off the surface of the lens, and also produce ectopion uveae and corectopia. Depending on the distribution of the synechiae, the pupil can be displaced to one side or pulled into a pear, oval, or slit shape. As the PAS become more extensive, IOP rises. The severity of the glaucoma is usually related to the extent of the synechiae. On occasion, elevated IOP is noted despite open angles; in this situation the membrane has covered the angle but not yet contracted to form permanent adhesions. The corneal endothelium may appear normal but more often has the appearance of tiny guttata. The cornea may become edematous when IOP is elevated.



Chandler’s syndrome
Chandler’s syndrome is the most common variant of the ICE syndrome. The most prominent features of Chandler’s syndrome are corneal endothelial dysfunction and corneal edema. The endothelium has a hammered silver appearance that is similar to, but less coarse than, the abnormalities seen in Fuchs’ dystrophy. On specular microscopy the endothelial cells appear pleomorphic with dark cytoplasmic areas and loss of the normal hexagonal patterns. Early in the course of the disease specular microscopy may demonstrate normal and abnormal endothelial areas. With time, the normal areas diminish in size. Specular microscopy also helps to distinguish early Chandler’s syndrome from posterior polymorphous dystrophy, which has some clinical similarities. The corneal endothelium becomes so dysfunctional that epithelial edema develops at normal or only slightly elevated IOPs. In contrast to the marked corneal changes, the iris involvement is generally mild and limited to superficial stromal dissolution. Corectopia is minimal or absent. Peripheral anterior synechiae form, but they are not as diffuse and do not extend as far anteriorly as in progressive iris atrophy. For this reason glaucoma is often mild.
Cogan-Reese syndrome
The Cogan-Reese, or iris nevus, syndrome is differentiated from progressive iris atrophy and Chandler’s syndrome by the occurrence of pigmented lesions of the iris. Some eyes have pedunculated iris nodules, other eyes have diffuse pigmented lesions, and still others have both. The pigmented iris lesions may appear years after the other features of the syndrome and then may disappear spontaneously. These are definitively not nevi: instead, they are islands of normal iris pinched by the contracting endothelial membrane. The iris may have any degree of dissolution from mild to severe. A similar variability is noted in the degree of corneal edema and the severity of the angle-closure glaucoma. Especially in the early stages, characteristics of both progressive iris atrophy and iris nevus syndrome may be seen in the same iris.
The differential diagnosis of the ICE syndrome is very large because the clinical features of the syndrome are so variable. Included are corneal conditions such as posterior polymorphous dystrophy and Fuchs’ dystrophy, iris abnormalities such as iridoschisis and malignant melanoma, developmental disorders such as Rieger’s syndrome and aniridia, and miscellaneous conditions such as neurofibromatosis and anterior uveitis with nodules. The diagnosis is often missed early because the corneal and iris signs may be subtle. Most of the conditions in the differential diagnosis are bilateral, so a unilateral condition should raise the possibility of the ICE syndrome.
Treatment
The treatment of the ICE syndrome is as variable as the clinical picture. If corneal edema produces pain or reduced vision, the patient may be helped by hypertonic solutions or soft contact lenses. In many cases corneal edema is improved if IOP is reduced by medical or surgical therapy. Some patients with the ICE syndrome eventually require penetrating keratoplasty.
Glaucoma is initially treated with the full range of medical therapy. Laser trabeculoplasty offers no help in this condition because most of the angle is covered by a membrane or sealed with synechiae. Short-term success has been reported with a goniotomy procedure. But as the entire angle is progressively covered by a membrane or sealed by synechiae, medical therapy or angle surgery eventually fail because of relentless angle closure. Filtering surgery or glaucoma drainage devices are often required to control glaucoma in patients with the ICE syndrome. However, clinicians should be aware that functioning filtering blebs often fail after 2–5 years, perhaps related to proliferation of a membrane over the internal opening of the sclerostomy, despite the use of adjunctive antimetabolite therapy. In such cases, some would attempt a repeat trabeculectomy with mitomycin application or tube operation. In most cases, corneal edema clears after successful filtering surgery; in other cases, edema persists, presumably because of corneal endothelial dysfunction. At one time it was proposed that eyes with the ICE syndrome undergo a wide basal iridectomy to prevent total closure of the angle by PAS. This approach has not proved to be useful. Ultimately, the discovery of the stimulus to epithelialization of the corneal endothelium will lead to inhibitors, and possibly prevent this difficult angle-closure disease.
POSTERIOR POLYMORPHOUS DYSTROPHY
Posterior polymorphous dystrophy is a disease of the corneal endothelium that is sometimes associated with glaucoma. This condition affects both eyes and is usually inherited as an autosomal dominant trait, although autosomal recessive patterns have been reported. Association with an abnormality on the long arm of chromosome 20 has been reported for at least one large pedigree. Posterior polymorphous dystrophy occurs without known racial or sexual predilections, although several Thai families have been reported with this condition in association with Alport’s syndrome.
Histopathology
Histopathologic study of eyes from individuals with posterior polymorphous dystrophy reveals a thin Descemet’s membrane covered by multiple layers of collagen. This is lined by a layer of cells that various investigators have stated resembles endothelium, epithelium, or fibroblasts. In some cases a membrane has been noted in the angle and on the anterior surface of the iris.
Pathogenesis
The cause of posterior polymorphous dystrophy remains controversial. Analogous to the ICE syndrome, some investigators postulate that a dysplastic corneal endothelium produces a basement membrane-like material that extends into the angle and onto the iris. When the membrane contracts, it causes iris atrophy, corectopia, and iridocorneal adhesions. Most authorities believe posterior polymorphous dystrophy is a developmental disorder; a few authorities have postulated that a viral infection, perhaps herpes simplex, causes metaplasia of the corneal endothelium. Perhaps several different stimuli can produce similar epithelialization of endothelium.
Clinical presentation
Although the clinical picture of posterior polymorphous dystrophy is quite variable, the most typical physical finding is a cluster or linear arrangement of vesicles in the posterior cornea surrounded by a gray haze. The deep corneal stroma and Descemet’s membrane may also have band-like thickenings, white patches, peau d’orange appearance, or excrescences that project into the anterior chamber. Posterior polymorphous dystrophy may also have associated corneal edema, iris atrophy, mild corectopia, and iridocorneal adhesions. Most cases of this syndrome are non-progressive, and the individuals affected maintain good vision throughout their lives. These eyes are often asymptomatic. The diagnosis is made on routine examination or because other members of the family have been affected.
A minority of the individuals with posterior polymorphous dystrophy develop progressive corneal changes including corneal edema. Glaucoma occurs in 10–15% of patients with this disorder. In some cases glaucoma occurs in eyes with iris atrophy, corectopia, and iridocorneal adhesions. However, in other cases glaucoma occurs in eyes with open angles and an anterior insertion of the iris into the ciliary body, which resembles congenital glaucoma.
The differential diagnosis of posterior polymorphous dystrophy includes Fuchs’ corneal dystrophy, congenital hereditary corneal dystrophy, Axenfeld’s syndrome or Rieger’s syndrome, and congenital glaucoma. These conditions should be distinguished readily by slit-lamp examination. The Haab’s striae of congenital glaucoma are thin areas surrounded by a thickened, retracted Descemet’s membrane. In contrast, the corneal involvement of posterior polymorphous dystrophy consists of thickened areas without breaks in Descemet’s membrane.
Treatment
Most cases of posterior polymorphous dystrophy require no treatment. If the cornea becomes edematous, the patient should be treated with hypertonic solutions, soft contact lenses, and penetrating keratoplasty as needed. The presence of iridocorneal adhesions is a risk factor for failure of keratoplasty. Eyes with glaucoma are treated with medication and then filtering surgery as necessary.
EPITHELIAL DOWNGROWTH
Pathophysiology
Epithelial downgrowth (also called epithelial ingrowth) occurs when an epithelial membrane enters an eye through a wound and then proliferates over the corneal endothelium, trabecular meshwork, anterior iris surface, and vitreous face ( Fig. 16–8 ). The epithelial membrane in the angle contracts, producing PAS and severe angle-closure glaucoma without pupillary block.


Cataract surgery is the most common cause of epithelial downgrowth. In the past it was estimated that this complication occurred in about 1 in 1000 ICCE cataract operations. A relatively recent review cites a 0.12% incidence decreasing to 0.08% in the decade of the 1980s. Furthermore, epithelial downgrowth was found in 8–26% of eyes enucleated for complications of cataract surgery. In recent years epithelial downgrowth has been encountered less commonly because of the wide adoption of microsurgery and better techniques of cataract extraction. Epithelial downgrowth has also been reported after penetrating keratoplasty, glaucoma surgery, penetrating trauma, and unsuccessful removal of epithelial cysts of the anterior segment.
In most cases the epithelium invades the eye through a fistula or a wound gape; fistulas have been detected in 23–50% of such eyes. It is also possible that epithelium can grow into a suture tract, or it can be introduced into an eye at the time of surgery or trauma. Epithelial downgrowth can occur after uncomplicated surgery but is more likely to occur if surgery is associated with hemorrhage, inflammation, vitreous loss, or incarcerated tissue. Other factors seem to be endothelial damage and stromal vascularization. In the past it was observed that epithelial downgrowth occurred more frequently when cataract surgery was performed with a corneal section rather than with a limbal section. Furthermore, many authorities believed that epithelial downgrowth was less common when the cataract wound was covered with a limbus-based conjunctival flap rather than with a fornix-based conjunctival flap. These observations came from uncontrolled studies, and their significance is not clear. Furthermore, modern cataract extraction techniques have markedly reduced the incidence but have not eliminated the problem.
Histopathology
Histopathologic study of biopsy specimens and enucleated globes reveals the presence of stratified squamous epithelium on the corneal endothelium, iris, and angle structures. The epithelium, which resembles conjunctival epithelium, is one to three cells thick except at the advancing corneal edge, where it may be five cells thick. However, the epithelium may also originate from cornea. The epithelial membrane passes posteriorly in some eyes to cover the ciliary processes, pars plana, and retina. If a fistula is present, it is also lined by stratified squamous epithelium.
Clinical presentation
Epithelial downgrowth usually is seen as a low-grade persistent postoperative inflammation, including conjunctival injection, photophobia, discomfort, and aqueous humor cells. Careful examination may reveal large whitish cells floating in the anterior chamber. Affected eyes often have some evidence of current or past wound leak and are hypotonic if the fistula is still functional. The key to diagnosing this condition is finding a grayish white membrane with a scalloped, thickened leading edge on the posterosuperior corneal surface. The cornea overlying the membrane may be edematous, and the iris may be drawn up to the old wound or incision. The anterior surface of the iris often appears compacted, with loss of its normal architecture. In advanced cases the eye is painful with bullous keratopathy and intractable glaucoma.
The diagnosis of epithelial downgrowth is usually made on clinical grounds as indicated above. Specular microscopy may be helpful in some cases, but this technique requires a clear cornea overlying the membrane. Involvement of the iris is dramatically demonstrated when it is treated with large, low-energy argon laser burns (200–500 μm, 100–400 mW, 0.1 second), which turn the epithelial membrane white. (Normal iris does not respond this way.) Because of the gravity of the situation, it is usually desirable to confirm the diagnosis histopathologically. Aqueous humor can be aspirated, passed through a Millipore filter, and then examined to identify epithelial cells. An alternative approach is to obtain a specimen by scraping a small portion of the posterior cornea with a blunt spatula. If these approaches are unfeasible or inadequate, biopsies should be taken of the posterior cornea and iris.
Most cases of epithelial downgrowth are associated with severe glaucoma. Usually the glaucoma is caused by a membrane that lines the angle and contracts to form PAS. Other factors contributing to the glaucoma include chronic inflammation, pupillary block, and obstruction of the trabecular meshwork by desquamating epithelial cells.
Treatment
The treatment of epithelial downgrowth is often difficult and unrewarding. All of the techniques in current use attempt to close the fistula and then to excise or destroy the epithelium in the eye. It is common to determine the extent of iris involvement using the argon laser as described above. The corneal portion of the membrane can then be destroyed with cryotherapy or chemical cauterization. The affected iris can be excised, and cryotherapy can be applied to any remaining membrane on the ciliary body and retina. An alternative approach is to do an en-bloc excision of all involved tissues. Others have excised the involved iris and vitreous with a vitrectomy instrument and destroyed any remaining membrane with cryotherapy after the eye has been filled with air. Yet another approach involves excision of involved tissues, a penetrating keratoplasty, and implantation of a glaucoma shunt. One case report using adjunctive 5–fluorouracil, both for glaucoma control and in an attempt to control the epithelialization, was unsuccessful. Many other techniques have been described in the past for treating epithelial ingrowth, including X-radiation, beta-irradiation, curettage with alcohol, and photocoagulation. These have been largely abandoned as ineffective. Immunotoxin has been shown to inhibit epithelial proliferation in tissue culture; perhaps an agent like this may find some use in the future in this very frustrating condition.
All of the current techniques have been reported to salvage some eyes with epithelial downgrowth and even to maintain good vision in a few cases. However, recurrences of the downgrowth are common, and surgery is frequently associated with complications that include corneal edema, chronic inflammation, macular edema, and phthisis bulbi. The results of treatment are better if the condition is diagnosed early. However, it must be stressed that preventing epithelial downgrowth is far more effective than treating established disease. Surgical and traumatic wounds must be cleaned of epithelial tissue fragments and foreign material and then sutured meticulously.
FIBROVASCULAR INGROWTH
Fibrovascular tissue can grow into an eye if there is an open wound after penetrating trauma or surgery. Fibrovascular ingrowth occurs more frequently if the trauma or surgery is associated with hemorrhage, inflammation, or incarcerated tissue. Fibrovascular ingrowth can occur from pars plana incisions, as well as from more anterior ones. In some cases the ingrowth resembles a vascular stalk that enters the eye through an old wound and then fans out over the anterior segment. In other cases the ingrowth forms a gray-white membrane posterior to the corneal endothelium, without an obvious entry site or a vascular stalk. The membrane may have an interlacing pattern of gray fibers that has been compared to woven cloth. Various authorities have attributed the invading fibroblasts to the subconjunctival connective tissue, corneal stroma, limbal tissue, and metaplastic endothelium. The invading fibrovascular tissue grows over the corneal endothelium, anterior iris surface, vitreous face, and angle, where it contracts to form PAS. On occasion the membrane can also attach to the retina and cause a traction detachment.
Fibrovascular ingrowth usually causes glaucoma when the membrane covers the angle and then contracts to form peripheral anterior synechiae. Other factors contributing to the glaucoma include uveitis, pupillary block, and underlying trauma. In many ways, fibrovascular ingrowth resembles epithelial downgrowth, although it is less virulent in its course.
It is far better to prevent fibrovascular ingrowth than to treat the condition once established. In the past this condition was a common finding in eyes enucleated after cataract surgery or trauma. With current microsurgical techniques, fibrovascular ingrowth is encountered far less often.
The glaucoma associated with fibrovascular ingrowth is usually managed by medical therapy. In some cases posterior glaucoma drainage device implantation or cyclophotocoagulation is required to control IOP. On occasion it is possible to excise the fibrovascular tissue, including the fistula at the old wound. However, this approach is not suitable for most eyes with fibrovascular ingrowth because the involvement of the anterior segment is too extensive. Furthermore, the poor visual prognosis usually does not warrant such aggressive surgery in most cases. Cyclodestructive procedures can often alleviate painfully high IOPs.
Glaucoma has been reported from proliferation of iris melanocytes across the angle and the remainder of the anterior segment. This type of glaucoma is extremely rare.
FLAT ANTERIOR CHAMBER
A flat anterior chamber after penetrating trauma or surgery can lead to the formation of PAS and secondary angle-closure glaucoma without pupillary block ( Table 16–1 ; Fig. 16–9 ). The development of synechiae is related to the duration of the flat anterior chamber and the degree of inflammation of the eye. There are a number of reports on delayed re-formation of the anterior chamber after cataract extraction. In most of these studies secondary angle-closure glaucoma was common if the anterior chamber was flat for 5 days or longer. Flat anterior chambers are encountered far less often with modern microsurgical cataract techniques. However, flat anterior chambers occur not uncommonly after filtering operations, and they are often allowed to persist for a few to several days before re-formation is attempted. Flat anterior chambers also may occur in association with malignant glaucoma and penetrating keratoplasty.
| Malignant Glaucoma | Choroidal Detachment | Pupillary Block | Suprachoroidal Hemorrhage | Wound Leak | |
|---|---|---|---|---|---|
| Central anterior chamber | Flat or shallow | Shallow | May be normal | Flat or shallow | Flat or shallow |
| Intraocular pressure | Normal or elevated | Low | Normal or elevated | Normal or elevated | Low |
| Fundus appearance | Usually normal | Large, smooth, brown mass | Usually normal | Dark brown or dark red elevation | Choroidals may be present |
| Suprachoroidal fluid | Absent | Present | Absent | Present | Absent |
| Relief by drainage of suprachoroidal fluid | No | Yes | No | Yes | No |
| Relief by iridectomy | No | No | Yes | No | No |
| Patent iridectomy | Yes | Yes | No | Yes | Yes |
| Onset | Usually within days, but may be months | Usually within first week | Anytime | Immediately or within first few days | Usually within first few days but may be late with adjunctive antimetabolites |
| Seidel test | Negative | Negative | Negative | Negative | Positive |

Following re-formation of a flat anterior chamber, the residual secondary angle-closure glaucoma is treated with standard medical therapy. Often patients respond better to medical treatment than would have been predicted by the extent of the angle closure. This suggests that some of the trabecular meshwork is functional behind the apparent PAS; that is, the synechiae are bridging rather than closing the angle if the duration of tissue approximation is short enough. If medical treatment is inadequate, a laser trabeculoplasty can be considered if at least one-third to one-half of the angle is open. However, the clinician must be aware that a sustained postlaser IOP rise may necessitate filtering surgery. Other alternatives include filtering operations, cyclodestructive procedures, and surgical goniosyneechialysis of the PAS.
INFLAMMATION
Inflammation can produce glaucoma through a variety of mechanisms, including increased viscosity of the aqueous humor, obstruction of the trabecular meshwork by inflammatory cells and debris, scarring of the outflow channels, elevated episcleral venous pressure, forward displacement of the lens–iris diaphragm, and pupillary block from posterior synechiae. Inflammation can also produce angle-closure glaucoma without pupillary block when the peripheral iris swells as a result of the inflammatory process, when precipitates or exudates in the angle contract to form PAS, or when there is forward rotation of the ciliary body. These can occur after surgery or trauma, in idiopathic inflammatory conditions, or with specific uveitis entities such as interstitial keratitis, sarcoidosis, ankylosing spondylitis, pars planitis, and juvenile rheumatoid arthritis, particularly the pauciarticular variety. Peripheral anterior synechiae form more readily in eyes with shallow anterior chambers and in eyes afflicted with chronic granulomatous inflammatory disease.
Secondary angle-closure glaucoma without pupillary block is usually managed with medical therapy. It is crucial that residual inflammation be suppressed with corticosteroids. However, the ophthalmologist must keep in mind the possibility of inducing corticosteroid glaucoma. Patients are often more comfortable with the addition of cycloplegic agents which may, by dilating the pupil, prevent pupillary block from posterior synechiae formation. Virtually all topical glaucoma agents are used to control IOP, with two guarded exceptions. Miotics may be helpful in the pseudophakic eye if it is quiet, but they are usually counterproductive in the presence of persistent inflammation. Similarly, prostaglandins should be used with caution because they may occasionally precipitate an inflammatory reaction. Hyperosmotic agents are administered on occasion for acute elevations of IOP.
If medical therapy fails to control IOP, filtering surgery must be considered. Because standard filtering surgery is less likely to be successful in inflamed eyes (and these patients are often young people), filtering surgery with adjunctive antimetabolite therapy or glaucoma drainage devices such as the Molteno, Baerveldt, or Ahmed implants should be performed. In children with inflammatory disease the prospects for successful filtering surgery are further reduced by rapid healing, low scleral rigidity, and the increased thickness of Tenon’s capsule. A few authorities have used a modified goniotomy procedure – trabeculodialysis – to treat children with inflammation and glaucoma. A goniotomy knife is used to depress the iris and lyse any PAS present. The trabecular meshwork is then incised below Schwalbe’s line, and the trabecular tissues are retracted further.
PENETRATING KERATOPLASTY
Angle-closure glaucoma can develop after penetrating keratoplasty, from mechanisms including pupillary block, postoperative inflammation, or a flat anterior chamber from a wound leak. The severity of the glaucoma is generally related to the extent of the synechial closure. The incidence of postkeratoplasty angle closure is reduced by performing one or more iridectomies, closing the wound meticulously, using a graft slightly larger than the recipient bed, and administering corticosteroids postoperatively. However, in some cases the iris becomes attached to the corneal wound, and it is pulled forward in progressive fashion. Glaucoma of one sort or another is a complication in about 20% of penetrating keratoplasties.
Most cases of postkeratoplasty angle-closure glaucoma without pupillary block can be managed with standard medical treatment. Many of these eyes are aphakic and respond well to cholinesterase inhibitors, β-blockers, α-adrenergic agonists, prostaglandins, and carbonic anhydrase inhibitors. If pupillary block is contributing to the glaucoma, a laser iridotomy should be performed. Laser trabeculoplasty may be helpful, provided that at least one-third to one-half of the angle is open. When medical treatment fails to control the glaucoma, filtering surgery with mitomycin-C can be successful if conjunctiva is not heavily scarred. However, the surgeon must proceed with care to avoid damage to the corneal graft. Filtering surgery alone often fails. A posterior glaucoma drainage device may also be very useful in these situations, though special care must be taken to place the tube within the anterior chamber far from the new graft tissue. Cyclocryotherapy and other ciliary body destructive procedures are used commonly to control IOP before or after penetrating keratoplasty. The pressure reduction is often temporary, but the procedure can be repeated as required.
IRIDOSCHISIS
Iridoschisis is a patchy dissolution of the iris in which the anterior stroma separates from the posterior stroma and muscle layer. The anterior stroma then splits into strands that project into the anterior chamber and sometimes touch the cornea. Iridoschisis is usually bilateral and tends to involve the lower iris quadrants. This condition usually occurs in older individuals but has been reported in children. Many patients have a pre-existing chronic ocular disease such as uveitis. Glaucoma occurs in about 50% of the patients and is usually related to the development of PAS in the region of the iris strands. Pupillary block and the release of pigment and debris may also contribute to the glaucoma in some cases. The cornea overlying the iris strands may develop bullous keratopathy. Angle closure may occur from forward bowing of the anterior iris stroma. One report suggests that angle-closure glaucoma may actually cause iridoschisis and that any patient with the condition should have primary angle-closure glaucoma ruled out as an underlying condition.
Elevated IOP and iridoschisis are usually managed by medical therapy. If pupillary block is playing a substantial role, a laser iridotomy should be performed. In some cases filtering surgery is required to control the glaucoma.
ANIRIDIA
Aniridia produces angle-closure glaucoma without pupillary block. This is discussed in the section devoted to glaucoma in infants and children in Chapter 23 .
POSTERIOR PUSHING (OR ROTATIONAL) MECHANISM
As discussed earlier, it is possible to conceptualize secondary angle-closure glaucoma without pupillary block as occurring through two major mechanisms: by anterior pulling or by posterior pushing, or rotation. In the anterior pulling mechanism, a membrane, exudate, or fibrous band in the angle contracts and pulls the iris forward into contact with the trabecular meshwork. In the posterior pushing mechanism, the peripheral iris is displaced by the lens, vitreous, or ciliary body (see Fig. 16–2 ). The posterior pushing mechanism is often accompanied by swelling and anterior rotation of the ciliary body, which further acts to close the angle. When the ciliary body swells, it rotates forward about its attachment at the scleral spur and diminishes the diameter of the ciliary ring. Both of these factors reduce the tension on the zonules and allow the lens to move forward.
Several mechanisms, often overlapping, have been proposed to explain the role of the ciliary body in ‘pushing’ forms of secondary angle-closure glaucoma:
- 1.
When the anterior uveal tract swells from inflammation or vascular congestion, the ciliary ring is narrowed, which reduces tension on the zonules, permits the lens to come forward, and displaces the peripheral iris.
- 2.
When the ciliary body swells, it also rotates forward about its attachment to the scleral spur, which again loosens the zonules and displaces the root of the iris. The ciliary body is like a fan that opens about its attachment at the scleral spur as it swells.
- 3.
Finally, ciliary body swelling is often accompanied by the accumulation of suprachoroidal and supraciliary fluid, which further rotates the ciliary body and iris root into the angle.
CILIARY BLOCK GLAUCOMA (AQUEOUS MISDIRECTION OR MALIGNANT GLAUCOMA)
In 1869 von Graefe described an uncommon complication of ocular surgery consisting of a postoperative flat or shallow anterior chamber and elevated IOP. He named this condition ‘malignant’ glaucoma because it relentlessly worsened despite conventional therapy. The term ‘malignant glaucoma’ is not related to the pathophysiology of the condition and is often frightening to patients who believe they have a malignancy of the eye. This text’s original author, Dr Robert Shaffer, strongly felt that the term malignant glaucoma should be replaced by the term ciliary block glaucoma.
The term ciliary block or malignant glaucoma refers to a spectrum of atypical angle-closure glaucomas that share several essential features. Other terms have been proposed for this condition, many of which purportedly point to the underlying pathophysiology. These terms include aqueous misdirection , hyaloid block glaucoma and posterior aqueous entrapment . Historically, this condition was commonly appreciated as a complication of a filtering procedure in eyes with pre-existing angle-closure glaucoma or shallow anterior chambers, although anomalous ‘triggers’ for its presentation have been reported, such as laser iridotomy, miotic usage, infectious endophthalmitis, retinal conditions, and hyperplastic ciliary processes.
There is good agreement in the literature about several essential features of this condition, but other features are more controversial. Clinically, ciliary block glaucoma is suspected in the presence of a Spaeth grade 1 or 2 shallow anterior chamber, with the prominent axial shallowing of the peripheral and central anterior chambers simultaneously. The pressure is usually higher than expected; in the early postoperative period it may simply be between 15 and 20 mmHg despite the appearance of what would seem to be an otherwise adequate bleb; in other cases the pressure can be quite high indeed.
To diagnose ciliary block glaucoma, it is essential to eliminate the possibility of pupillary block ; hence a patent iridectomy must be established before this diagnosis can be considered. Sometimes the diagnosis is made only in retrospect, after evaluating the eye’s response to several interventions. For example, cycloplegics can ameliorate malignant glaucoma and miotics can exacerbate the situation. If surgical intervention is necessary, disrupting the hyaloid face or collapsing the vitreous is usually curative; some use aggressive vitrectomy/lensectomy to form a unicameral eye creating easy passage for fluid from the vitreous cavity into the anterior chamber.
Other aspects that are sometimes seen with ciliary block glaucoma include the rarity of spontaneous resolution – and hence its ‘malignant’ designation. It is usually bilateral in predisposition, and it is often worsened by conventional glaucoma surgery such as iridectomy or filtration procedures. The clinical presentation of ciliary block glaucoma is similar to that of other conditions, notably angle-closure glaucoma with ciliary choroidal detachment syndrome. This specific condition is usually bilateral in its presentation, with identifiable choroidal detachments associated with other diseases, such as HIV ( Fig. 16–10 ).

Though not well described in the classical literature on malignant glaucoma, recent authors have observed the accumulation of fluid in the suprachoroidal space in some cases of ciliary block glaucoma, which can frequently be confirmed by ultrasonic biomicroscopy (UBM) ( Fig. 16–11 ). This finding has been incorporated into a model that proposes the common denominator of choroidal effusion as underlying several of the angle-closure glaucomas (see below).

Other situations that may overlap with the appearance of ciliary block glaucoma include eyes that have undergone cataract extraction, with or without lens implantation, with sequestration of aqueous behind the iris plane. These conditions have been referred to as ‘iridovitreal block’ and ‘retrocapsular aqueous misdirection’.
The pathophysiologic sequence of ciliary block glaucoma is thought to be as follows. After some initiating event (e.g., shallowing of the chamber during trabeculectomy) there is cause for misdirection of the aqueous to circulate into or behind the vitreous body. This apparently leads to an alteration of the vitreous volume and its compaction, with a cycle of increasing vitreous swelling and reduced conductivity of aqueous anteriorly. The enlarging vitreous body is unable to exchange aqueous across the hyaloid face at the junction of the zonules, vitreous face, and ciliary processes. This progressive vitreal engorgement results in shallowing both axially and peripherally in the anterior chamber, with increasing apposition of the peripheral iris into the angle, setting up a further cycle of angle-closure glaucoma. A recent model proposes that choroidal expansion (proposed as an initiating event in acute angle closure as well) may also be a contributory event for anterior vitreal movement in malignant glaucoma, and hence its frequent clinical association with primary angle-closure glaucoma.
The management of ciliary block glaucoma needs to be sequential, and specific to the anatomy of the presenting eye. It is essential to eliminate the possibility of pupillary block glaucoma by verifying or creating a patent iridectomy. Miotic medications should be discontinued, and vigorous cycloplegia (atropine 1% twice daily plus phenylephrine 2.5% four times daily) as well as the use of topical steroids (prednisolone 1% six times daily) should be instituted. Other agents to reduce aqueous production, such as topical α-adrenergic agonists, β-blockers, carbonic anhydrase inhibitors, prostaglandins, or osmotic agents, can be used to reduce the pressure. A waiting period of approximately 5 days has been advised, if feasable, with an intensive medical regimen to see if there is resolution, with as many as half of the cases resolving during this interval.
In the event that surgical intervention is necessary, the status of the lens largely determines which non-medical options are available. Either a needle aspiration of vitreous through the pars plana or pars plana vitrectomy can be curative in phakic eyes (see Fig. 36-15 ). Eyes that have had cataract extraction – with or without a lens implant – and a retained posterior capsule permit the frequently curative intervention of the Nd:YAG laser *
* Only rarely, in the days of large surgical iridectomies, was adequate visualization possible of the ciliary processes or hyaloid to attempt either YAG or argon laser therapy in phakic eyes.
for direct incision of the hyaloid face. (This may be the mechanism of argon laser shrinkage of visible ciliary processes: peripheral disruption of the hyaloid–ciliary interface.) In an eye with a retained posterior capsule , as with a posterior chamber intraocular lens, (see Fig. 36-16 ), it is necessary to sequentially eliminate pupillary block, retrocapsular block, and hyaloid block by respectively lasering through the iris, posterior capsule, and hyaloid face. In the acapsular eye (e.g., aphakia) (see Fig. 36–17 ), hyaloidectomy centrally and peripherally can be undertaken with the Nd:YAG laser or with incisional surgery.Vigorous surveillance is still necessary in all malignant glaucoma eyes, even after laser or surgery, because recurrent cases of ciliary block glaucoma have been reported. This has been seen particularly after vitreous aspiration or vitrectomy, which may not have been sufficiently anterior in the phakic eye to access and interrupt the obstruction of the anterior hyaloid face, so close to the lens. In such instances it may be necessary to sacrifice the lens to access the hyaloid itself. Chronic atropine drops may be needed; and great attention should be paid to the fellow eye, which is at a high risk for recapitulating the events of the first eye’s ciliary block glaucoma attack.
INTRAOCULAR TUMORS
Ocular malignant melanoma is frequently associated with glaucoma through a variety of mechanisms, including direct extension of the tumor into the trabecular meshwork, seeding of tumor cells into the outflow channels, obstruction of the meshwork by pigment or pigment-laden macrophages, neovascularization, PAS, iridocyclitis, and hyphema. Benign iris and ciliary cysts have been associated with glaucoma, presenting in ways similar to the plateau iris mechanism. Melanomas of the choroid and ciliary body can also displace the lens–iris diaphragm and produce angle-closure glaucoma without pupillary block. In most cases, glaucoma occurs when the melanoma is already large, and enucleation is the appropriate therapy. There have been a few reports of surgery for angle-closure glaucoma in eyes that were determined later to harbor undetected melanomas. At times, iris melanomas invade the angle and cause secondary angle closure without pupillary block ( Fig. 16–12 ). Generally these eyes have good vision, and the glaucoma is managed by medical treatment.