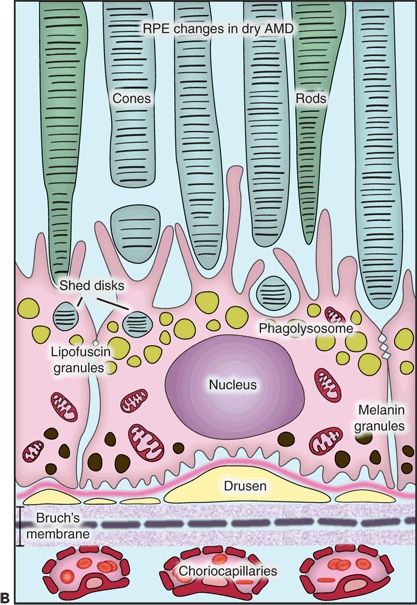
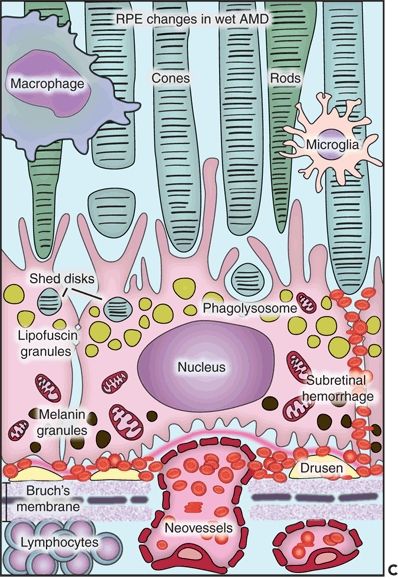
Figure 5.1 Histopathological changes in the two main forms of AMD. This diagram is showing the structural and functional changes of a normal RPE cell when developing dry or wet AMD. A. The RPE in a young retina has a homogenous distribution of the melanin granules, a BM without deposits, and a preserved function. B. In dry AMD, the RPE melanin granules are localized basally, and lipofuscin granules increase in number while the efficacy of the disks digestion reduces. Note the formation of drusen between the basement membrane of the RPE and the inner collagen layer of the BM and the thickened BM. C. In wet AMD, CNV develops, and an inflammatory response develops with an influx of lymphocytes and macrophages to the scene. These fragile new vessels leak and lead to subretinal hemorrhage.
The most common histologic findings in nonneovascular or dry AMD are drusen; a thickened and basophilic BM; and geographic atrophy, hypertrophy, and hyperplasia of the RPE. Choroidal neovascularization (CNV), pigment epithelial detachment (PED), and disciform scar are important features of neovascular or wet AMD. The purpose of this chapter is to give a detailed description of the histopathologic findings encountered in AMD with emphasis on the ultrastructure, composition, and formation of different lesions.
NORMAL AGING OF THE RETINA
In discussing the histopathology of AMD, one must consider normal aging changes in the outer retina, a major risk factor for the development of this disease. The photoreceptor layer, RPE, BM, and choriocapillaris constitute the Ruysch’s complex (1). This complex suffers age-associated changes that commence at approximately the age of 20.
Certainly, the RPE shows ultrastructural changes seen in electron microscopy. Its melanin granules migrate to the basal portion of the cell, and the amount of lipofuscin granules increases as a result of the phagocytosis of the rod and cone outer segments. With age, the efficacy of rod and cone outer segment digestion reduces, and they can be extruded into the BM and in the space between the basement membrane of the RPE and its cellular membrane where they accumulate. Lipids from the digestion of organelles like mitochondria and endoplasmic reticulum can also deposit here (2).
Lipofuscin is an autofluorescent pigment formed through the oxidation of unsaturated fatty acids. In short, it is an undigestable residue of cytoplasmic catabolism. Studies showed that content of lipofuscin in the cells increases with age. On the contrary, melanin content decreases showing an inverse relation between these two compounds not only in their final concentration but also in their topographical distribution. This interesting finding may suggest a protective mechanism in the formation of lipofuscin (3).
Thickened BM is another alteration that occurs over time. Histologically, there is an increase in basophilia with hematoxylin and eosin (H&E) stain because of progressive calcium salt and lipid deposition in both inner and outer collagenous zones (4). Moreover, studies have shown that a large portion of the lipids found in this tissue are lipoprotein-like particles that increase with advancing age. These deposits were found to increase in the inner collagenous and elastic layers, but not in the outer collagenous layer. Authors have related these findings to the formation of a hydrophobic barrier within the BM that altered the filtration capacity through the area (5). These deposits may also be related to small granules, another major deposit found at the BM by Huang et al. They suggested there could be an interaction between this two inclusion types, but the identity of these small granules could not be determined (6). In addition, the accumulation of cholesterol esters with advancing age can be compared to accumulation of cholesterol esters in the intima of large arteries in atherosclerosis (7). Finally, there is an increase in collagen cross-linking in the inner portion of this membrane causing a negative effect on its permeability. This cross-linkage provides strength and density to the collagen network together with the loss of flexibility, elasticity, and filtration properties. Therefore, the RPE collagenases become less effective in the removal of other BM components considering this tight network is blocking their way (8).
The photoreceptor layer manifests a different response to aging. Immunohistochemical analysis concludes that the cone photoreceptor population shows more anomalies compared with rod photoreceptor population in non-AMD retinas. Nevertheless, the signs of degeneration have an early onset in cones, but rods appear to die more promptly. Cones can prolapse into the outer plexiform layer and subretinal space and lose their synaptic contact without succumbing to cell death as do rods (9).
Some authors consider AMD to be an accentuation of normal aging changes. Age is a key factor in its pathogenesis. Nevertheless, many people maintain an excellent vision in spite of advancing age and its consequences.
DRY/NONNEOVASCULAR AMD
Drusen
Drusen are deposits of extracellular matrix localized between the basement membrane of the RPE and the inner collagen layer of the BM. They can extend to the outer collagenous zone if there is discontinuity in the central band of elastic fibers (10,11). They are often seen in the aging retina, as a hereditary condition (dominantly inherited drusen) or secondary to a variety of intraocular processes including inflammation, trauma, and chronic retinal detachment (2). However, an increase in number, size, and confluence was established as a risk factor for the development of AMD (12). Because of this, it is important to distinguish which yellow deposits will lead to atrophy of the RPE or CNV. The American Academy of Ophthalmology proposed the following classification according to the size of the drusen (13):
- Small (less than 64 μm of diameter)
- Intermediate (64–124 μm of diameter)
- Large (≥125 μm of diameter)
Immunohistochemical and proteomic studies of drusen composition have revealed different proteins other than RPE remnants (lipofuscin) like immunoglobulins, class II antigens, acute-phase proteins (e.g., fibrinogen, C-reactive protein, and vitronectin), components of the complement cascade and its inhibitors, apolipoproteins B and E, and lipids (e.g., cerebrosides), among others (8,14,15). These deposits are collectively known as drusen, but they are not all alike. Drusen main types are hard, soft, and diffuse or confluent, although authors have also described several others subtypes such as basal, nodular, mixed, and calcified regressing drusen (11).
Hard drusen are pinpoint-sized, sharp-edged yellowish retinal lesions easily seen on ophthalmoscopic exam. They are hyperfluorescent in the early stages of the fluorescein angiography and stain late without leakage. Histopathologically, hard drusen are hyaline-like, dense, rounded, homogenous bodies restricted to the basement membrane of the RPE and BM. They consist of a uniform periodic acid-Schiff-positive material and are eosinophilic on the H&E stain (Fig. 5.2A and B) until they start accumulating calcium and consequently become basophilic (16). They also stain lightly positive with special staining technique for lipid (2). On the electron microscopy, they are finely granular or amorphous nodules with similar electrodensity to the basal membrane of the RPE. They may also contain pale vesicles, tubular structures, curly membranes, and wide-banded collagen (15). The overlying RPE is thinned due to degenerative changes as described in Ulshafer et al. (17) electron microscopy studies. There are several hypotheses concerning the formation of hard drusen. Some authors have shown that the main initiating event in their development is the shedding of portions of RPE cells into the BM that are then hyalinized during the apoptosis process (18,19). Others have demonstrated that they are formed by lipidization and degeneration of single cells (20,21). This matter is still uncertain.
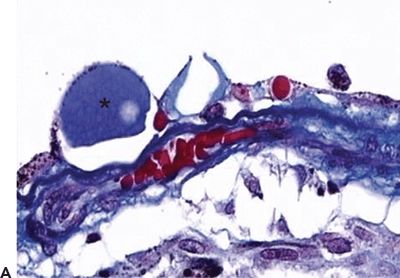
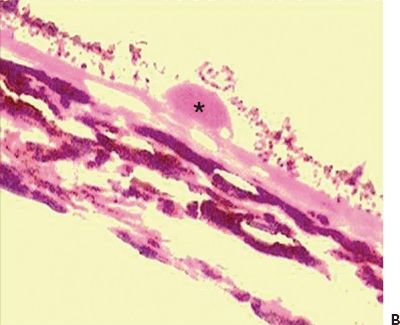
Figure 5.2 Histopathologic section of drusen (asterisk) showing atrophy of the overlying pigment epithelium and photoreceptors. A. Masson trichrome digital stain 40×. B. H&E stain 20× (Courtesy Zárate JO, Alvarado M© 2011. Unpublished data.)
In contrast, soft drusen have less well-defined boundaries and tend to become confluent. Histologically, they are composed of a pale-staining amorphous more irregular and granular material located in the thickened inner layer of the BM. Green et al. (16) pointed out that they represent a serous detachment of the thickened inner aspect of the BM along with the RPE. On electron microscopy, this thickened area consists of vesicles, membranous debris, and wide-spaced collagen associated with the basal laminar deposits (22).
The basal deposits represent one of the earliest morphologic changes in the AMD. The two main types of basal deposits are basal laminar deposits (BLamDs) and basal linear deposits (BLinDs) (23). BLamDs are seen as soft granular eosinophilic material or extensive plaques that elevate the atrophic RPE from the inner surface of BM. On electron microscopy, these deposits are found to be limited to the plasma membrane and the basement membrane of the RPE and are comprised of extracellular matrix material with wide-spaced collagen (24). These deposits contain laminin, collagen type IV, vitronectin, extracellular matrix–modulating metalloproteinases, activated complement, glycoproteins, heparan sulfate, cholesterol, carbohydrates, and apolipoproteins B and E (8,25). In contrast, BLinDs are located in the thickened inner layer of the BM and appear to be electron dense rich in lipid material. These deposits represent what many authors have described as “diffuse drusen” (26). With progressive death of pigment epithelial cells, drusen start to fade by decreasing in size and becoming what some authors called calcified drusen (27). In fact, RPE atrophy, represented by hypopigmentary changes on clinical exam, is seen concomitantly with calcified or refractile drusen in histopathologic studies (28).
In summary, soft drusen along with basal linear deposits reveal an RPE dysfunction that influences the development of CNV (29,30). It has been reported that the thickening due to these deposits predisposes the BM to splitting and consequent complications such as retinal PED, neovascularization, and scarring (31).
Geographic Atrophy
Geographic or areolar atrophy also called advanced “dry” AMD involves an extensive degeneration (150 μm or larger) of the RPE and the overlying receptors that are metabolically dependent upon the RPE. It is labeled as “geographic” because the areas of atrophic RPE are well demarcated and not related to any specific anatomy structure (32). Some authors describe a nongeographic atrophy in cases where atrophic areas are noncontiguous or manifest as speckled depigmented areas (13). On funduscopic examination, geographic atrophy is seen as a sharply demarcated round or oval hypopigmented spot, frequently found either juxtafoveal or parafoveal, that allows an enhanced view of the underlying large choroidal vessels (1,33). On fluorescein angiography, geographic atrophy demonstrates an early hyperfluorescent area due to the window defect in the RPE. The loss of epithelium transmits fluorescein brightly, but the extent of the lesion does not change in the late phases.
On microscopy, areas of geographic atrophy lack photoreceptors and RPE cells, and the outer plexiform layer is adherent to the BM (Fig. 5.3A and B) (4). This is seen as hypopigmented or atrophic areas overlying a diffusive thickened inner aspect of the BM (26). Calcified or retractile drusen, as previously described, are also observed (28). The RPE demonstrates other changes such as hypertrophy and hyperplasia, which are clinically seen as focal hyperpigmentation (23). Clumps of pigmented cells in the subretinal space and the outer retinal layers may also be observed histologically (26).
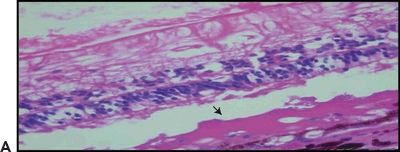
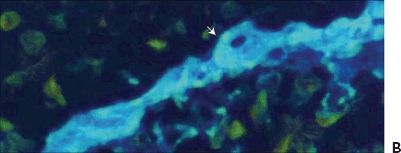
Figure 5.3 Geographic atrophy. A. Retinal and choroidal tissue. Photoreceptor lesion with fibrosis (arrow) H&E stain 20×. B. Macular retinal atrophy and fibrosis (arrow). Masson trichrome digital stain 60×. (Courtesy Zárate JO, Alvarado M© 2011. Unpublished data.)
Studies performed by Schatz et al. demonstrated that in addition to RPE atrophy, one may also observe atrophy of the choriocapillaris. During fluorescein angiography, the choriocapillaris fills more slowly than normal indicating an ultrastructural damage (32). It is not understood if the choriocapillaris atrophy is secondary to RPE deterioration or vice versa. In fact, there is direct in vitro evidence that RPE cells may support the survival of the choriocapillaris endothelial cells by both insoluble molecules and growth factors such as basic fibroblast growth factor (bFGF). In other words, without them, the cells become apoptotic (34). Therefore, the absence of RPE or anything that can reduce its availability, like deposits in BM, produces choriocapillaris atrophy. An alternative theory postulated by Ciulla et al. (35) supporting the vascular pathogenesis of AMD hypothesizes that there is a primary vascular alteration in the choroid, which eventually causes a collateral effect on the RPE. This issue requires further research.
Even though at one time it was assumed patients with geographic atrophy were not at risk of developing exudative complications, it is now well known that neovascular changes can occur (16). It has been established that this new vessel formation depends on the viability of the RPE so that it can only grow in the edges of the lesion where the pigment epithelium remains intact or hypertrophic (27). Furthermore, Kliffen et al. illustrated this event with a light microscopic image and demonstrated that the junctional zone was comprised of hypertrophic RPE cells and basal laminar deposits. They assumed that if neovascularization was present, this was where it was supposed to be found in association with macrophages and giant cells (36). Also, geographic atrophy can coexist with subretinal neovascularization or be followed by serous detachment of the RPE (11).
Pigment Epithelial Detachment
After a while, basal laminar deposits enlarge and coalesce providing a larger cleavage plane in BM, which is no longer seen as soft drusen but as a serous PED. Indeed, this appears to be a key event in the evolution to the development of subretinal neovascularization (16).
The inner aspect of the BM also suffers from rip or tears as a common complication of PEDs. The clinical and fluorescein angiographic aspects of these lesions were first described by Hoskin et al. in 1981. They determined that the tear always took place at the edge of the PED and that the remaining detached pigment epithelium retracted, leaving an area of nude BM. The retracted epithelium was folded parallel to the edge of the rip and, in some cases, reattached afterward to the BM at a new site. There was no modification on the overlying retina. Moreover, in the fluorescein angiogram, the nude BM made the choroidal vessels be seen in the first frame prior to hyperfluorescence within 2 seconds. There was no leakage into the subretinal fluid, but some late dye accumulation was identified subretinally as well as dark hypofluorescent folds of residual detached pigment epithelium (37). Tears may also develop after laser photocoagulation or antiangiogenic therapy in patients with neovascular AMD (38,39).
WET/EXUDATIVE/NEOVASCULAR AMD
Choroidal Neovascularization
Choroidal also known as subretinal neovascularization (CNV) is one of the main features of wet AMD. The new vessel formation extends from the choroid into the thickened portion of the BM or from the margins of the optic nerve head (40). Studies have confirmed that a prior break in the BM is not necessary for vessel proliferation because the new vessels can make their way through it by themselves (41). CNV is often seen in retinas in which softening and confluence of drusen have taken place (42). In histologic sections, Albert et al. (11) described the following findings: (a) breaks in BM; (b) a granulomatous inflammatory pattern with macrophages, lymphocytes, and fibroblasts; (c) basal laminar deposits and soft drusen; and (d) RPE depigmentation, hypertrophy, hyperplasia, and folding. On electron microscopy, both mature and newly formed capillaries have fenestrations similar to their choriocapillaris precursors. They also have high permeability to fluorescein and may cause leakage or fluorescein blockage (43). Even though they are initially very small, are nondetectable, and have capillary characteristics, they acquire arterial and venous features with time. Ophthalmoscopically, they can either be seen through the atrophic RPE or hidden underneath fibrous grayish tissue or RPE hypertrophy. CNV can be localized to the macula, peripapillary retina, or peripheral retina. CNV can be restricted to the BM (Fig. 5.4A and B) or trespass the RPE and grow into the subsensory space associated with fibroblastic RPE metaplasia and migration of macrophages (33). Anastomosis between choroidal and retinal vessels can develop. This characteristic is often seen in late stages of AMD when the disciform scar is well established (2). The neovascular membranes can be recognized in the fluorescein angiogram as a fine network of vessels with early filling, associated with exudation and subretinal hemorrhage. These specific elements are toxic for the RPE and photoreceptors, and they may lead to RPE detachment, proliferation of fibrous tissue, cystoid macular edema, and occasional circinate retinopathy (33,44,45). In this setting, fibroblasts can be identified invading the hematoma. Also, the surviving RPE cells are transformed into elongated spindly cells while the overlying photoreceptor atrophy resulting in a submacular nodule, plaque, or fibroglial scar tissue that contracts and distorts the retina (40).
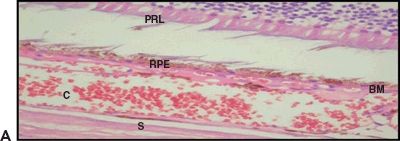
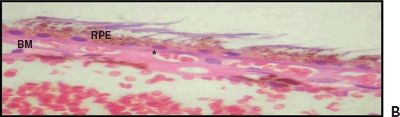
Figure 5.4 Wet AMD. A. Histologic section shows CNV at the BM. Retinal pigment epithelium (RPE), choroid (C), and sclera (S). H&E stain 40×. B. Magnified view of the choroidal neovessels (asterisk). H&E stain 60× (Courtesy Zárate JO, Alvarado M© 2011. Unpublished data.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


